FDA Investigator Robert D Tollefsen
Robert D Tollefsen has conducted inspections on 116 sites in 18 countries as of 04 Dec 2017. See below for a list of the FDA enforcement documents resulting from those inspections.
Investigator Details
Number of Inspected Sites:
116
Last Inspection Date:
04 Dec 2017
Investigator Role:
FDA Investigator
Redica ID:
Countries of Inspections:
Jordan,
United States of America,
Sweden,
India,
Germany,
Ireland,
Australia,
Belgium,
China,
United Kingdom of Great Britain and Northern Ireland,
Japan,
Spain,
Oman,
Canada,
Israel,
France,
Switzerland,
Singapore
FDA Investigators that have inspected at least one site in common with Robert D Tollefsen:
Aaron P Wozniak,
Adam R Cooke,
Ademola O Daramola,
Aditi S Thakur,
Alan P Kurtzberg,
Alberto A Viciedo,
Alexander M Kay,
Alice S Tsao,
Alicia M Mozzachio,
Alla Kachko, PhD,
Amalia C Himaya,
Amanda L Fyles,
Amanda S Zorn,
Amir Alavi,
Amy C Jordan,
Amy L Singer,
Amy M Cramer,
Amy N Chen,
Ana Delp Cintron,
Ana P Barido,
Anastasia I Offordile,
Anastasia M Shields,
Andrace L Deyampert,
Andrea A Branche,
Andrea D Swingle,
Andrea George, PhD,
Andrew K Haack, PhD,
Angel K Sandhu,
Angela E Glenn,
Anh M Lac,
Anissa M Cheung,
Anissa M Vargas,
Anita Narula, PhD,
Anita R Michael,
Ankur C Patel,
Ann L Demarco,
Ann Marie Montemurro,
Ann Marie Schofield,
Anna Lazar,
Anthony F Lorenzo,
April P Shaw,
Arie C Menachem,
Arsen Karapetyan,
Ashar P Parikh,
Ashleigh P Barkans,
Astrida B Mattson,
Atul J Agrawal,
Audrey Thereset Uy,
Azza Talaat,
Barbara J Rincon,
Barbara J Wilcox, PhD,
Barbara Janine Breithaupt,
Barbara Jwilimczyk Macri,
Barbara M Frazier,
Barry Cherney, PhD,
Bei Y He,
Benjamin J Smith,
Bichsa T Tran,
Bill Tacket, Jr,
Binh T Nguyen,
Bo Chi, PhD,
Bradley Dworak, PhD,
Brenda L Reihing,
Brenda W Uratani, PhD,
Brentley S Collins,
Brian D Nicholson,
Brian S Keefer,
Brien C Fox,
Brittny C Cargo,
Bruce H Mccullough,
Bryan Baker,
Bryce A May,
Burnell M Henry,
Byungja E Marciante,
Calvin B Koerner,
Carl A Anderson,
Carl Lee,
Carla J Lundi,
Carla Lankford, MD, PhD,
Carole L Jones,
Carolyn A Renshaw,
Carrie A Hughes,
Carrie Ann Plucinski,
Caryn M Everly,
Caryn M Mcnab,
Catherine J Laufmann,
Cathleen A Carr Sharpe,
CDR Cecily Jones,
CDR Ileana Barreto Pettit,
CDR Jeremy L Wally, PhD,
CDR Rochelle B Young, RPh, MSA,
CDR Thomas R Berry, PPh,
Celeta S Coves,
Celia L Hutchinstein,
Chad R Burger,
Chao Ming Tsai,
Charanjeet Jassal,
Charisse K Green,
Charles D Brown,
Charles I Ann,
Charles L Zhou,
Charles R Cote, RIC,
Cheron M Portee,
Chiaochun J Wang,
Chris A Sack,
Christian D Lynch (CDL),
Christina C Santos,
Christina J Sauder, PhD,
Christina V Santos,
Christine A Harman, PhD,
Christine M Cerenzio,
Christine M Humphrey,
Christopher D Leach,
Christopher D Rush,
Christopher J Adams,
Christopher R Czajka,
Christopher T Middendorf,
Clinton J Lott,
Concepcion Cruz, Jr,
Connie P Rezendes,
Constantin Y Philopoulos,
Corrine M Carter,
Craig A Garmendia,
Craig D Zagata,
Creighton T Tuzon,
Crystal A Harlan,
Crystal Monroy,
Cynthia J Lee, MS,
Cynthia Jim, CSO,
Cynthia L Kelley,
Dandan Wang, PhD,
Daniel J Brown,
Daniel J Lahar,
Daniel J Roberts,
Daniel W Cline,
Darla J Christopher,
Darren S Brown,
Daryl A Dewoskin,
Dave Hafner,
David A Paterson,
David Frucht, MD,
David G Eng,
David J Gomes,
David J Hafner,
David K Glasgow,
David M Beltran,
David Perkins,
Davinna Ligons, PhD,
Dawn E Barkans,
Dawn M Mccabe,
Debara R Reese,
Debara Reese,
Deborah A Greco,
Deborah Hammond,
Deborah S Hammond,
Debra I Love,
Debra J Bennett,
Debra L Pagano,
Demario L Walls,
Demitria J Xiradakis,
Denise L Burosh,
Denise M Digiulio,
Dennis Cantellops Paite,
Dennis E Guilfoyle, PhD,
Dennis G Kawabata,
Dennis M Farley,
Derek S Smith, PhD,
Destry M Sillivan,
Devaughn Edwards,
Devon M Shoop,
Deyaa Shaheen,
Dhaval H Patel,
Diana M Rand,
Diane Cvan Leeuwen,
Diane L Raccasi,
Dianna D Wardlow Dotter,
Dien N Nguyen,
Dina A Tallman,
Dina K West,
Dipesh K Shah,
Dmitriy V Volokhov, PhD,
Doan T Nguyen, PharmD,
Dolores E Price,
Don H Bark, PhD,
Donald C Obenhuber, PhD,
Donald L Lech,
Donna Ltartaglino Besone,
Doreen P Gubbay,
Douglas A Campbell,
Douglas C Kovacs,
Dr. Barbara D Paul, PhD,
Dr. Carmelo Rosa,
Dr. Chunchang Fang,
Dr. Jason R Caballero,
Dr. Mark J Seaton, PhD,
Dr. Ralph M Bernstein, PhD,
Dr. Robert C Horan, MD,
Dr. Sriram Subramaniam, PhD,
Dr. Willie F Vann, PhD,
Dr. Zhihao Qiu (Peter), PhD,
Dustin R Abaonza,
Dyvette Arline,
Edmund F Mrak, Jr,
Edward D Harris,
Edward Deberry,
Edward E Lockwood (EEL),
Edwin Melendez,
Eileen A Liu,
Eileen J Bannerman,
Elizabeth B Griffin,
Elizabeth S Howell,
Ellen Huang,
Emest F Bizjak,
Emily J Orban,
Emily Shacter,
Emmanuel Jramos Maldonado,
Ephrem Hunde, PhD,
Eric C Fox,
Eric L Dong, BS,
Eric M Mueller, PharmD,
Eric M Padgett,
Eric Rothschild,
Erika V Butler,
Erin D Mccaffery,
Erin L Mcfiren,
Esther C Broner, PhD,
Fabian Nchaparro Rodriguez,
Farhana Khan,
Felix Maldonado,
Frances Namuswe, PhD,
Francis A Guidry,
Gabriel R Mclemore,
Gajendiran Mahadevan, PhD,
Gam S Zamil,
Gene D Arcy,
Gene R Gunn,
George J Flynn,
George Lunn, PhD,
George Pyramides,
Gerald B Seaborn, Jr,
Gerald Feldman,
Gerald M Feldman, PhD,
Gerald N Mcgirl, DDS,
Gerard Pde Leon,
Gianine E Tompkins,
Ginger M Sykes,
Gloria J Baca, MS,
Grace E Mcnally,
Graham N Giesen,
Guerlain Ulysse,
Hai Lient Phung,
Hala L Selby,
Haley H Seymour,
Hamet M Toure, PharmD MPH,
Haroon Vohra (NMI),
Haruhiko Murata,
Hasan A Irier, PhD,
Heika R Bounds,
Heika R Tait,
Helen B Ricalde,
Helen Verdel,
Henry K Lau,
Huiquan Wu,
Hung H Do, MS,
Ibad U Khan,
Iris C Macinnes,
Ivis L Negron,
J David Doleski,
J Elkins,
Jacek Cieslak, PhD,
Jacob G Lutz,
Jacqueline Mdiaz Albertini,
James A Liubicich,
James D Hildreth,
James D Planchon,
James I Giefer,
James L Dunnie, Jr,
James M Kewley,
James M Mason,
James P Stumpff,
James R Evans,
James R Fleckenstein,
James S Stuart, BS,
James W Plucinski,
Jane M Kreis,
Jason F Chancey,
Jawaid Hamid,
Jean Blackston Hill,
Jean Lhu Primmer,
Jeanne Fringer, PhD,
Jee Chung, PhD,
Jeffrey A Sommers,
Jeffrey D Meng,
Jeffrey M Watson,
Jeffrey P Raimondi,
Jeffrey R Wooley,
Jeffrey W Shrifter, DPM,
Jen Sui,
Jennifer A Kemp,
Jennifer D Hollstrom,
Jennifer Gogley,
Jennifer L Bridgewater,
Jennifer L Huntington,
Jennifer Lalama,
Jennifer M Gogley,
Jennifer M Menendez,
Jennifer Macmillan,
Jessica L Pressley,
Jessica L Walters Moell,
Jessica M Monteiro,
Jianming Li,
Joan A Loreng,
Joan Adamo,
Joan T Briones,
Joanne E King,
Joanne Hosting,
Joanne M Hajoway,
Jodi M Gatica,
Joel D Hustedt,
Joel D Martinez,
Joel Welch, PhD,
Joey V Quitania,
Jogy George,
Johann M Fitch,
John A Gonzalez,
John Burr,
John D White,
John F Cipollo,
John R Myung,
Jolanna A Norton,
Jorge L Guadalupe,
Jose A Lopez,
Jose Acruz Gonzalez,
José E Meléndez,
Jose M Cayuela,
Jose Martinez, Jr,
Jose R Hernandez,
Jose R Lopez,
Joseph A Piechocki,
Joseph D Gong,
Joseph E Duran,
Joseph George,
Joseph Kutze, PhD,
Joseph P Iser, MD,
Joseph R Strelnik,
Joshua J Silvestri,
Joshua S Hunt,
Joy Rkozlowski Klena,
Jude C Dikes,
Julia Ventura,
Julian C Hanson,
Julianne C Mccullough,
Julie D Bringger,
Junho Pak,
Justin A Boyd,
Ka L Wong,
Kaarin M Slotte,
Kalavati Suvarna, PhD,
Karen A Briggs,
Karen C Daugherty,
Karen E D'orazio,
Karen M Montgomery (KMM),
Karen Takahashi,
Kari M Johansen,
Karlton T Watson,
Karyn M Campbell,
Katherine E Jacobitz,
Katherine Szestypalow,
Kathleen B Mihalik,
Kathleen B Swat,
Kathleen D Culver,
Kathleen R Jones, PhD,
Kathryn A Krentz,
Kathryn Carbone, MD,
Kathryn E King,
Keegan B Mixon,
Keith O Webber, PhD,
Keith Webber, PhD,
Kelli F Dobilas,
Kelli Regente,
Kellia N Hicks,
Kelly D Sheppard,
Kelly N Kerr,
Kelvin Cheung,
Kelvin X Sanders,
Kendra A Biddick,
Kenneth M Gordon,
Kenneth O Gee, PhD,
Kevin D Kallander,
Kevin P Foley,
Kham Phommachanh,
Kim Lthomas Cruse,
Kimberley A Hoefen,
Kip J Hanks,
Kirsten L Vadheim, PhD,
Ko U Min,
Kouros Kangarli,
Krishna Ghosh, PhD,
Kristen D Evans,
Kristin M Abaonza,
Kristy A Zielny,
L'oreal F Walker,
Lakisha M Williams,
Lance Mde Souza, MBA,
Lance Sindo, CIH, CQA, RS,
Lane V Christensen, PhD,
Larry K Austin,
Larry K Hampton,
Latorie S Jones,
Laura Fontan, MS,
Laurel A Beer,
Lauren Iacono Connors, PhD,
Laurie B Frazier,
Laurie Graham,
Laurie P Norwood,
Lawrence Y Lee, PhD,
LCDR Chad N Thompson,
LCDR Debra Emerson,
LCDR Jennifer H Rhyu,
LCDR Margaret Edi Gennaro,
LCDR Matthew J Morrison,
LCDR Michael H Tollon,
Lei Zhang, PhD,
Leigh Anne Myers,
Leiyun Boone, PhD,
Leonard H Lavi,
Lequita M Mayhew,
Lesley K Satterwhite,
Leslie D Wagner,
Li Li,
Liatte Kreuger, PharmD,
Libia M Lugo,
Liming Zhang,
Linan Ha, PhD,
Linda F Murphy,
Linda K Weir,
Linda R Kuchenthal,
Linda S Leja,
Linda Thai,
Lindsey M Schwierjohann,
Linh Tu,
Lisa C Lewis,
Lisa Hayka,
Lisa K Capron,
Lisa M Bellows,
Lisa M Feola,
Lisa M Parsons,
Loretta J Jordan,
Lori J Silverstein,
Lori S Lawless,
Lorie S Hannappel,
LT Daveta L Bailey,
LT John M Mastalski,
Lucila B Nwatu,
Luella J Rossi,
Luis A Carrion,
Luis A Dasta,
Lynda L Perry, PhD,
Mabel M Lee,
Madushini Dharmasena, PhD,
Mai X Huynh,
Maida Henesian (NMI),
Marc A Jackson, Jr,
Marcellinus D Dordunoo,
Marcia B Williams,
Marcus F Yambot,
Margaret M Annes,
Margarita Santiago,
Maria Joselopez Barragan, PhD,
Maria Pkelly Doggett, MBA,
Maria V Price,
Marian E Major, PhD,
Marie A Fadden,
Marie F Morin,
Marie K Kinkade,
Marijo B Kambere, PhD,
Marion Michaelis,
Mariza M Jafary,
Mark C Saale,
Mark E Chan,
Mark I Schwartz,
Mark R Mcclain,
Mark W Babbitt,
Marlene G Swider,
Marsha W Major,
Marshalette O Edwards,
Martin K Yau, PhD,
Marvin D Jones,
Mary Jeanet Mcgarry,
Massoud Motamed,
Matt D Suedkamp,
Matthew B Casale,
Maurice M Sheehan,
Maxine H Wong,
Maxwell Van Tassell, PhD,
Maxyne T Lam,
Maya M Davis,
Megan A Haggerty,
Meisha R Sampson,
Meisha Waters,
Melanie G Warzala,
Melanie M Walker,
Melanie W Pishnery,
Melina Lrodriguez Upton,
Melissa B Libby,
Melissa J Garcia,
Melissa T Roy,
Mercy Oyugi,
Meredith L Sheridan,
Michael A Charles,
Michael C Rogers,
Michael Curbarg,
Michael D Garcia,
Michael F Skelly, PhD,
Michael Gurbarg,
Michael J Lackey,
Michael J Vardon,
Michael P Sheehan,
Michael R Goga,
Michael R Klapal,
Michael S Araneta,
Michael Serrano,
Michael Shanks, MS,
Michael T Cyrus,
Michele D Thompson,
Michele L Forster, PhD,
Michele L Glendenning,
Michele L Obert,
Michele Perry Williams,
Michelle A Marsh,
Michelle Rfrazier Jessen, PhD,
Michelle Yclark Stuart,
Miguel A Martinez Perez,
Mihaly S Ligmond,
Mikel T Wright,
Milos Dokmanovic, PhD,
Min Shanmabel Liu,
Mohsen Rajabi Abhari, FDA,
Monica J Wilkins,
Montgomery Montgomery, Karen M,
Mra Mcculloughj,
Mra V Millarw,
Muna Algharibeh,
Muralidhara B Gavini, PhD,
Myra K Casey,
Nadeem I Chaudhry,
Nailing Zhang,
Nancy A Bellamy,
Nancy A Saxenian Emmons,
Nancy E Byerly,
Nancy F Scheraga,
Nancy G Schmidt,
Nancy M Espinal,
Nasir Ali, PhD,
Natalia Pripuzova, PhD,
Nataniel Phillips Sylvain, PhD,
Navista C Bolton,
Nawab A Siddiqui,
Nayan J Patel,
Neali H Lucas,
Nealie C Newberger,
Nibin Varghese, MS,
Nicholas F Lyons,
Nicholas L Hunt,
Nicholas L Paulin,
Nicholas Obiri, PhD,
Nicola M Fenty Stewart,
Nicole A Lloyd,
Nicole E Knowlton,
Nicole K Trudel,
Niketa Patel,
Norman K Starks,
Omotunde O Osunsanmi,
Pankaj H Amin,
Parul M Patel,
Patricia A Cochran,
Patricia F Hughes, PhD,
Patricia H Dlugosz,
Patrick B Cummings,
Patrick D Stone, MS,
Patsy J Domingo,
Paul F Rader,
Paul J Teitell,
Paul L Bellamy,
Paul Lihong Yeh, PhD,
Paul Rader,
Paul W Keller,
Paul Z Balcer,
Paula A Trost,
Penny H Mccarver,
Peter C Chow,
Peter E Baker,
Peter Kessler, PhD,
Peter S Diak,
Philip J Boston,
Phillip M Pontikos,
Phung Thien Nguyen,
Prabhu P Raju,
Pratik S Upadhyay, DDC,
Priscilla M Pastrana,
Qiao Y Bobo,
Qing Joanna Zhou, PhD,
R Edwadebey Deberry,
Rafael E Arroyo,
Rafael Nevarez Nieves,
Rafeeq A Habeeb,
Ralph A Erickson,
Ramon E Martinez,
Randa Melhem, PhD,
Randall N Johnson,
Randy L Self,
Raymond T Oji,
Rebecca E Dombrowski,
Rebecca K Olin,
Rebecca Rodriguez,
Rebecca T Davis,
Regina T Brown,
Reyes Candau Chacon, PhD,
Richard E Needham,
Richard H Penta,
Richard Heath Coats,
Richard K Vogel,
Richard L Friedman,
Richard Ledwidge (nmi), PhD,
Richard W Thornton,
Richmond K Yip,
Riley C Myers, PhD,
Rita K Kabaso,
Robert B Shibuya, MD,
Robert C Coleman,
Robert Darius,
Robert J Ham,
Robert Jennings,
Robert M Barbosa,
Robin Levis, PhD,
Rocco C Black,
Rochelle K Kimmel,
Roger F Zabinski,
Rona Leblanc, PhD,
Ronald L Koller,
Ronda Leblanc,
Rose Ashley,
Rowena S Nguyen,
Roy R Rinc,
Rumany C Penn, PharmD,
Russell K Riley,
Ruth Moore, PhD,
S Lori Brown, PhD MPH,
Sachinkumar V Patel,
Saied A Asbagh,
Saleem A Akhtar,
Samantha J Bradley,
Samuel K Gibbons, Jr,
Sandra A Boyd,
Sandra A Hughes,
Sandra S Saniga,
Sangeeta M Khurana, PhD,
Sangeeta M Rataul,
Santiago Gallardo Johnson,
Santos E Camara,
Sara H Gabel,
Sarah Arden,
Sarah B Tanksley,
Sarah E Mcmullen,
Sarah E Rhoades,
Satheesh Thomas,
Scott A Nabe,
Scott E Norris,
Scott N Lim,
Scott R Nichols, PhD,
Scott T Ballard,
Sean R Marcsisin,
Seneca D Toms,
Serge L Beaucage,
Shafiq S Ahadi,
Shari H Shambaugh,
Sharon K Thoma, PharmD,
Sheila Dreher Lesnick,
Sheilyn H Huang,
Shelby N Turner,
Shelley H Beausoleil,
Shererk,
Sherri J Jackson,
Sherri N Rohlf, MD,
Shuang Tang,
Shuen G Chai,
Sidney B Priesmeyer,
Simone E Pitts,
Sinai I Davis,
Sneha S Patel,
Sonia R Peterson,
Sonya M Edmonds,
Sridhar Thumma,
Stacey S Degarmo,
Stanley Au,
Stephanie A Slater, MS,
Stephanie D Crockett,
Stephanie Mangigian, MS/OSH, RN,
Stephanie T Durso,
Stephen D Brown,
Stephen J Mottola,
Stephen L Beekman,
Stephen R Souza,
Steven A Gonzales,
Steven A Rubin,
Steven C Madzo,
Steven D Kehoe,
Steven Eastham,
Steven Fong, MS, PhD,
Steven M Weinman,
Steven P Donald,
Stuart W Russell,
Sue Lee Chan,
Sunitha K Rajaram, PhD,
Susan D Yuscius,
Susan F Laska, MS,
Susan M Corrales,
Susan M Jackson,
Susan M Turcovski,
Susan P Bruederle,
Susan T Hadman,
Susan W Ting,
Suyang Qin,
Suzanne N Vallez,
Syed N Ali,
Taichun Qin, PhD,
Tajah L Blackburn,
Tamika White,
Tammy L Chavis,
Tania Y Hall,
Ted L Anderson,
Temar Q Williams,
Teresa I Navas,
Terrance L Thomas,
Terri L Dodds,
Thai D Truong,
Thao T Kwan,
Thao X Tran,
Thea C Grome,
Thomas J Arista,
Thomas W Gordon,
Thuy T Nguyen, LCDR,
Timothy C Grome,
Timothy T Kapsala,
Tina M Pawlowski,
Tina S Roecklein,
Todd M Stankewicz,
Tonia F Bernard,
Toyin B Oladimeji,
Tracey L Harris,
Tracy K Li,
Truong Xuan Nguyen (Andy),
Uduak M Inokon,
Unnee Ranjan,
Uttaniti Limchumroon (Tom),
Vanessa R Muller,
Vanessa Y Gelsey, PhD,
Vanessa Y Jacobs,
Verlinda A Narcisse,
Veronica Fuentes, MS,
Vibhakar J Shah, PhD,
Vioela J Caze,
Virgilio F Pacio, CSO,
Viviana Matta,
Vlada Matusovsky,
Walden H Lee,
Wayne E Seifert,
Wayne T Smith,
Wei Wang, PhD,
William D Tingley,
William F Lagud, Jr,
William Hallett, PhD,
William J Leonard,
William V Millar,
Xianghong Jing (Emily), PhD,
Xiao Wang,
Xiaohan Cai, PhD,
Xiaojun Yan,
Xiaokuang Lai, PhD,
Xing Wang, PhD,
Xiomara Copeland,
Yangmin Ning,
Yasamin Ameri,
Yetao Jin, PhD,
Yi Wang, PhD,
Yong Hu,
Yumi J Hiramine,
Yvesna C Blaise,
Yvette I Johnson,
Yvonne C Mcknight,
Yvonne C Wilkes,
Yvonne E Lozano,
Zachary A Bogorad,
Zachary L Miller,
Zachary L Stamm,
Zakaria I Ganiyu,
Zhongren Wu,
Ziyang Su, PhD
Robert D Tollefsen's Documents
Experience Redica Systems’ NEW investigator profiles and dashboards
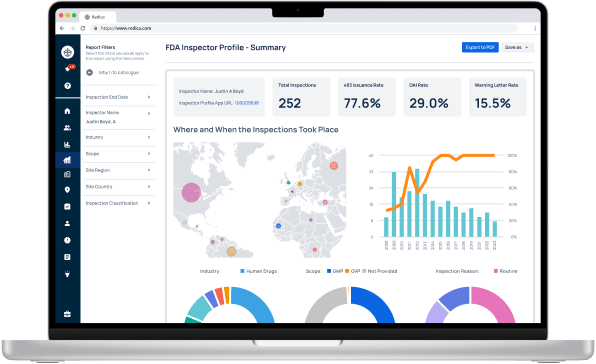
Redica Systems customers get access to over 1,400 FDA Investigator profiles, containing powerful analysis like:
- Total inspections conducted
- 483 rate
- OAI rate
- Warning Letter rate
- Inspections by Industry, Scope, and Inspection Reason
- And much more
Investigator Profiles are not sold as on-demand documents. If you’d like to see one, fill out the form below to connect with our Sales Team and get it in your inbox as little as 24 hours.
Talk to sales to access our investigator profiles
Loading...
Investigator Profiles Are Just the Tip of the Iceberg
Becoming a Redica Systems customer not only provides you with unlimited access to profiles similar to the one above for every current and former FDA Investigator, but also grants you access to:
Inspection Intelligence
- Pre-Approval Inspection (PAI) trends
- The ability to filter nearly any FDA enforcement action by GxP labels like GMP, GCP, etc.
- Latest 483s: see what the trends are across all FDA Investigators
- CFR Heatmap: Citations aggregated by Subpart by Year
Vendor Intelligence
- Full inspection histories for your vendors down to the specific site level
- All of the functionality mentioned above but isolated to just your vendors
Regulatory Intelligence
- Zero in on the trends for a specific agency/regulator (ex. FDA, MHRA, EMA, Health Canada)
- All the latest "Signals" from agencies around the world, segmented by Country, Type (ex. Guidance, Decree...), Category (ex. Rules/Regulations/Guidance, News, Reports...), Theme (ex. Cell and Gene Therapy, AI...), and more