FDA Investigator Shafiq S Ahadi
Shafiq S Ahadi has conducted inspections on 185 sites in 23 countries as of 25 Apr 2024. See below for a list of the FDA enforcement documents resulting from those inspections.
Investigator Details
Number of Inspected Sites:
185
Last Inspection Date:
25 Apr 2024
Investigator Role:
FDA Investigation Participant
Redica ID:
Countries of Inspections:
United States of America,
Germany,
China,
Japan,
Denmark,
Croatia,
Belgium,
United Kingdom of Great Britain and Northern Ireland,
Singapore,
Malaysia,
Dominican Republic,
Spain,
Korea (Republic of),
France,
Italy,
Brazil,
Costa Rica,
Canada,
Netherlands,
Ireland,
Czechia,
Sweden,
Mexico
FDA Investigators that have inspected at least one site in common with Shafiq S Ahadi:
Abraham M Maekele,
Adaliz Santaliz Cruz,
Adam R Cooke,
Adam S Freeman,
Adree N Anderson,
Akbar J Zaidi,
Alan L Truong,
Alan M Barker,
Alan M Roberts,
Alicia M Mozzachio,
Althea A Williams,
Amanda Dinaro,
Amy H Ruble,
Amy M Cramer,
Ana L Kewes,
Ana Paulap Sandee,
Anders W Evenson,
Andrew A Hoopes,
Andrew A Leboeuf,
Andrew J Barrowcliff,
Angela Jourdain,
Angela K Shen,
Anthony R Bucks,
April K Hill,
Ashley A Mutawakkil,
Babatunde D Babalola,
Barbara I Rogolsky,
Barbara M Frazier,
Bei Y He,
Benjamin J Dastoli,
Bill Tacket, Jr,
Billy M Battles,
Binh T Nguyen,
Bonnie E Conley,
Bonnie E Pierson,
Brent T Hall,
Brentley S Collins,
Brett R Havranek,
Brian D Nicholson,
Brittani N Franklin,
Brittany D Terhar,
Bruce H Mccullough,
Bryan S Roddy,
Burnell M Henry,
Candace S Tucker,
Carl A Huffman, III,
Carlos M Gonzalez,
Carmen Y Fisher,
Carolyn E Barney,
Carrie Ann Plucinski,
Catherine J Laufmann,
CDR Sean T Creighton,
CDR Thomas R Berry, PPh,
Ceisha C Ukatu,
Chad E Schmear,
Chad W Rice,
Charles L Larson,
Charles O'brein,
Cherie T Parker,
Cherrie A Zachary,
Cheryl D Mccall,
Christian D Lynch (CDL),
Christina L Bigham,
Christina V Santos,
Christine A Harman, PhD,
Christine M Parmentier,
Christopher D Leach,
Christopher T Peters,
Claire M Minden,
Clifford F Long,
Clotia Cabbey Mensah,
Cody D Rickman,
Conner N Mann,
Creighton T Tuzon,
Cynthia Jim, CSO,
Cynthia M Goudeau,
Damaris Y Hernandez,
Dana D Carter, SR DDS,
Danial S Hutchison,
Daniel C Kearns,
Daniel J Lahar,
Darrah,
David A Foran,
David A Place,
David F Graham,
David J Eide,
David J Gasparovich,
David K Glasgow,
David M Beltran,
David Perkins,
Dawn C Olenjack,
Deborah M Trout,
Decarlos A Gomez,
Dennis R Butcher,
Dennis R Hock,
Destry M Sillivan,
Devaughn Edwards,
Dewayne E Whitlock,
Dina K West,
Dolores Harper,
Donald C Obenhuber, PhD,
Donald L Myers,
Donna Ltartaglino Besone,
Dr. Robert C Horan, MD,
Dustin K Hampton,
Edward E Lockwood (EEL),
Emilio O Escobar,
Eric A Breselow,
Eric C Fox,
Eric C Nielsen,
Eric M Mueller, PharmD,
Eric M Padgett,
Eric S Pittman,
Eric T Huebler,
Eric W Anderson,
Erika V Butler,
Erin C Hill,
Erin Hill (EH),
Esther A Ofori,
Farhana Khan,
Fernando C Martinez,
Francis J Eng,
Frank J Marciniak,
Fred K Kelly,
Gabriel R Mclemore,
Gene D Arcy,
Georgia A Layloff,
Gideon N Esuzor,
Gilbert Valdez, RS,
Gisselle I Sensebe,
Gloria J Champagne,
Grace I Cortesini,
Gretchen Lf Trendel,
Gwyn G Dickinson,
Heath W Cartwright,
Hector A Carrero,
Hector Jcolon Torres,
Helen B Ricalde,
Helen Y Saccone,
Howard Anderson, PhD,
Howard Sklamberg,
Jacob G Lutz,
Jacqueline Mdiaz Albertini,
Jacqueline S Warner,
James A Beaulieu,
James D Hildreth,
James D Planchon,
James I Giefer,
James K Rice,
James M Odonnell,
James P Stumpff,
James R Evans,
James R Fleckenstein,
James W Leonette,
James W Plucinski,
Janae D Price,
Janet B Abt,
Janet Pulver,
Janete F Guardia,
Janna C Hutchinson,
Jared P Stevens,
Jason F Chancey,
Jawaid Hamid,
Jay Slater, MD,
Jazmine N Still,
Jean Lhu Primmer,
Jeanne J Chiu,
Jeffery A Hangartner,
Jeffery L Sumter,
Jeffrey B Moody,
Jennifer C Johnson,
Jennifer Cahill,
Jennifer D Hollstrom,
Jennifer L Bridgewater,
Jesse A Vazquez,
Jessica L Peterson,
Jimmie L Weigand,
Joan A Loreng,
Jocelyn C Turner,
Jocelyn E Massey,
Jocelyn E Sparks,
Jocelyn T Ramos,
John A Iwen,
John L Stevens,
John M Mcinnis,
John M Seale,
Johnson,
Jonathan R Campos,
Joseph A Morkunas,
Joseph A Piechocki,
Joseph A Seitz, III,
Joseph D Gong,
Joseph F Owens,
Joseph George,
Joseph R Lambert,
Julie D Bringger,
Julie V Bailey,
Kamara Mo,
Kara L Roden,
Karen C Daugherty,
Karen Emasley Joseph,
Karen M Montgomery (KMM),
Katherine E Jacobitz,
Katherine M Taylor,
Kathleen B Swat,
Kathleen D Culver,
Kathleen S Tormey,
Kellia N Hicks,
Kelly D Sheppard,
Kelly K Nachtigal,
Kelvin Cheung,
Ken L Bowers,
Kenneth D Bland,
Kenneth S Boehnen,
Kevin D Kallander,
Kham Phommachanh,
Kim M Downing,
Kimberly Lewandowski Walker,
Kirtida Patel,
Kristi B Panzer,
Lance Mde Souza, MBA,
Larry K Hampton,
Laura S Huffman,
Laurel A Beer,
Lauren E Skokan,
Lawrence Carter, III,
LCDR Debra Emerson,
LCDR Matthew J Morrison,
Leighton Kn Gai,
Leo J Lagrotte,
Leonard H Lavi,
Lequita M Mayhew,
Lewis K Antwi,
Lillian C Hsu,
Lillian Hsu,
Lindsey Brown, PhD,
Linhart,
Lionell L Thomas,
Lisa Hornback,
Lisa L Flores,
Lisa L Gilliam,
Lisa M Lopez,
Lori A Carr,
Lori S Lawless,
LTJG Bradley E Benasutti,
Luis Soto Lopez,
Lydia S Chan,
Maida Henesian (NMI),
Maksim A Levenchuk,
Malgorzata G Norton, PhD,
Malini Wileman, PhD,
Margaret A Smithers,
Margaret Torres Vazquez,
Marie F Morin,
Marion Michaelis,
Mariza M Jafary,
Marlo Ianm Alintanahin,
Mary A Millner,
Mary K Concannon,
Mary L Schuckmann,
Matthew H Hunt,
Matthew M Schuckmann,
Matthew R Sleeter,
Maxwell Van Tassell, PhD,
Megan M Jekel,
Meisha Waters,
Melanie M Walker,
Melanie W Pishnery,
Melissa J Holz,
Michael A Feingold,
Michael C Zubelewicz,
Michael G Mayfield,
Michele L Forster, PhD,
Michele L Glendenning,
Michele L Obert,
Michele Perry Williams,
Michelle J Glembin,
Michelle Yclark Stuart,
Mihaly S Ligmond,
Minerva Rogers,
Minh D Phan,
Miranda D Mcdonald,
Monica Cburgos Garcia,
Monica E Murie,
Monica M Mcclure,
Nancy G Schmidt,
Nancy P Hamburg,
Nancy T Waites,
Natalya M Ananyeva,
Natasha N Mccants,
Natasha R Johnson,
Neal L Adams,
Nibin Varghese, MS,
Nicholas J Presto,
Nicholas P Diorio,
Nicholas Z Lu,
Nicole J Conklin,
Nicole S Williams,
Nobuko H Katagiri,
Norman K Starks,
Nydia E Colon,
Olga Simakova, PhD,
Omotunde O Osunsanmi,
Pamela L Velez Vega,
Pamela N Agaba,
Pankaj H Amin,
Paola S Barnett,
Patricia A Mcilroy,
Patricia L Payne,
Patrick C Klotzbuecher,
Patrick L Wisor,
Paul E Stein,
Paul L Bellamy,
Paul R Whitby,
Paula A Trost,
Peng Zhou,
Penny H Mccarver,
Perry H Gambrell,
Peter M Boyt,
Philip J Boston,
Phung Thien Nguyen,
Prabhu P Raju,
Pradip N Akolkar,
Rachel T Evans,
Rafael Padilla,
Ralph H Vocque,
Randy L Clarida,
Raymond W Brullo,
Regina T Brown,
Reginald D Neal,
Reyes Candau Chacon, PhD,
Rhonda Alexander,
Richard H Penta,
Richard Heath Coats,
Richard Jay Tucker, RS,
Richard L Rutherford,
Richard W Koller,
Rita K Kabaso,
Robert C Coleman,
Robert D Tollefsen,
Robert J Ham,
Robert Jennings,
Robert R Wilson,
Roberto Macina,
Robin K Reel,
Rosanna M Goodrich,
Rose Ashley,
Russell K Riley,
Samuel K Gibbons, Jr,
Sarah E Morris,
Sarah E Rhoades,
Saundrea A Munroe,
Scott A Golladay,
Scott T Ballard,
Seneca D Toms,
Sergey S Akimov,
Sergio Chavez (NMI),
Shanna R Haden,
Sharon K Thoma, PharmD,
Shaun M Olson,
Shawn B Johnson,
Shelton L Stribling,
Sheri L Stephenson,
Sherry G Bous,
Shirley J Berryman,
Shirshendu K Deb, PhD,
Sidney B Priesmeyer,
Simone E Pitts,
Sondra L Phillips,
Stephanie T Durso,
Stephen C Mahoney,
Stephen D Brown,
Stephen D Eich,
Steven D Kehoe,
Steven P Donald,
Steven W Brown,
Susan D Yuscius,
Susan M Jackson,
Susan P Bruederle,
Susanna E Ford,
Tamala P Bogan,
Tamala P Magee,
Tamara Brey,
Tamika D Cathey,
Tammara A Stephens,
Tara L Breckenridge,
Tara L Greene,
Tara L King,
Thai T Duong,
That Q Dang,
Thomas J Arista,
Thomas O Morgan,
Thomas R Stanley,
Thomas R Withers,
Thuy T Nguyen, LCDR,
Tiara Nbrown Crosen,
Timothy C Grome,
Todd Q Dang,
Tony T Yang,
Torrey M Ward,
Travis E Chapman,
Trevor A Parker,
Unnee Ranjan,
Valeria A Moore,
Verlinda A Narcisse,
Veronica Fuentes, MS,
Vicky C Stoakes,
Victoria A Wagoner,
Vijaya L Simhadri,
Viviana Matta,
Walsworth,
Warren J Lopicka,
Wayne D Mcgrath,
Wendy R Blame,
William C Corley,
William D Bassett, Jr,
William R Chang,
Xiaojun Yan,
Xiuju Lu,
Yaodong Huang,
Yiping Jia,
Yun Wu, PhD,
Yvonne E Lozano,
Zachary A Bogorad,
Zhong Li, PhD,
Zi Qiang Gu
Shafiq S Ahadi's Documents
Experience Redica Systems’ NEW investigator profiles and dashboards
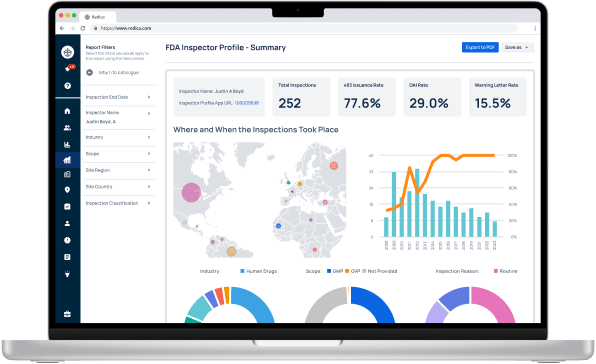
Redica Systems customers get access to over 1,400 FDA Investigator profiles, containing powerful analysis like:
- Total inspections conducted
- 483 rate
- OAI rate
- Warning Letter rate
- Inspections by Industry, Scope, and Inspection Reason
- And much more
Investigator Profiles are not sold as on-demand documents. If you’d like to see one, fill out the form below to connect with our Sales Team and get it in your inbox as little as 24 hours.
Talk to sales to access our investigator profiles
Loading...
Investigator Profiles Are Just the Tip of the Iceberg
Becoming a Redica Systems customer not only provides you with unlimited access to profiles similar to the one above for every current and former FDA Investigator, but also grants you access to:
Inspection Intelligence
- Pre-Approval Inspection (PAI) trends
- The ability to filter nearly any FDA enforcement action by GxP labels like GMP, GCP, etc.
- Latest 483s: see what the trends are across all FDA Investigators
- CFR Heatmap: Citations aggregated by Subpart by Year
Vendor Intelligence
- Full inspection histories for your vendors down to the specific site level
- All of the functionality mentioned above but isolated to just your vendors
Regulatory Intelligence
- Zero in on the trends for a specific agency/regulator (ex. FDA, MHRA, EMA, Health Canada)
- All the latest "Signals" from agencies around the world, segmented by Country, Type (ex. Guidance, Decree...), Category (ex. Rules/Regulations/Guidance, News, Reports...), Theme (ex. Cell and Gene Therapy, AI...), and more