FDA Investigator Terri L Dodds
Terri L Dodds has conducted inspections on 74 sites in 16 countries as of 12 Jun 2006. See below for a list of the FDA enforcement documents resulting from those inspections.
Investigator Details
Number of Inspected Sites:
74
Last Inspection Date:
12 Jun 2006
Investigator Role:
FDA Investigator
Redica ID:
Countries of Inspections:
France,
Australia,
Hungary,
Switzerland,
United States of America,
Netherlands,
United Kingdom of Great Britain and Northern Ireland,
Italy,
Ireland,
Germany,
Spain,
Puerto Rico,
Belgium,
Canada,
Croatia,
Japan
FDA Investigators that have inspected at least one site in common with Terri L Dodds:
Adam R Cooke,
Alan P Kurtzberg,
Alexander M Kay,
Alexandra B Pitkin,
Alexandria L Capuano,
Alicia M Mozzachio,
Amalia C Himaya,
Amanda L Fyles,
Amir Alavi,
Ana M Rivera,
Anastasia M Shields,
Anderson,
Andrea A Branche,
Andrew K Haack, PhD,
Andrew S Limson,
Angela E Glenn,
Anita Narula, PhD,
Anna Lazar,
Anthony A Charity,
April L Young,
Arie C Menachem,
Ariel Cruz Figueroa,
Arsen Karapetyan,
Ashar P Parikh,
Astrida B Mattson,
Azza Talaat,
Babajide Michael Osunsanmi,
Barbara J Rincon,
Barbara Janine Breithaupt,
Barbara Jwilimczyk Macri,
Barry Cherney, PhD,
Benjamin J Dastoli,
Benjamin S Liao,
Bichsa T Tran,
Binh T Nguyen,
Bo Chi, PhD,
Bonita S Chester,
Bonnie E Conley,
Bonnie E Pierson,
Bradley Dworak, PhD,
Brenda L Reihing,
Brenda W Uratani, PhD,
Brian J Ryan,
Brian P Putz,
Brien C Fox,
Brittany D Terhar,
Bruce H Mccullough,
Bryan A Galvez,
Burnell M Henry,
Calvin B Koerner,
Carl A Anderson,
Carl A Huffman, III,
Carl Lee,
Carla J Lundi,
Carlos A Medina,
Carolina D Vasquez,
Carrie A Hughes,
Caryn M Everly,
Caryn M Mcnab,
Cassandra L Abellard,
Catherine J Laufmann,
Cathleen A Carr Sharpe,
CDR Rochelle B Young, RPh, MSA,
Cecilia H Kieu,
Charisse K Green,
Charles R Cote, RIC,
Chelsea N Sealey,
Cheron M Portee,
Christian D Lynch (CDL),
Christina C Santos,
Christina V Santos,
Christine A Harman, PhD,
Christopher R Czajka,
Comyar Shoghi,
Connie P Rezendes,
Crystal A Harlan,
Cynthia J Lee, MS,
Cynthia Jim, CSO,
Cynthia L Gorveatt,
Dandan Wang, PhD,
Daniel J Moskowitz,
Daniel J Roberts,
Daniel R Solis,
Daniel W Cline,
David A Oluwo,
David J Daworski,
David J Gomes,
David Serrano,
Davinna Ligons, PhD,
Dawn E Barkans,
Deborah A Greco,
Dennis Cantellops Paite,
Devaughn Edwards,
Devon M Shoop,
Diane Cvan Leeuwen,
Diane R Weidley,
Diane T Wade, MS,
Doan T Nguyen, PharmD,
Dogbeda F Mackenzie,
Dolores E Price,
Don H Bark, PhD,
Donna Ltartaglino Besone,
Donna Mwilliams Hill, PhD,
Douglas A Campbell,
Douglas C Kovacs,
Dr. Carmelo Rosa,
Dr. Chunchang Fang,
Dr. Zhihao Qiu (Peter), PhD,
Dyana K Stone,
Edmund F Mrak, Jr,
Edward D Mcdonald,
Edwin Melendez,
Edwin Ortega,
Eileen A Liu,
Elaine A Bunch,
Elena Karnaukhova, PhD,
Elizabeth Ll Edwards,
Elizabeth S Howell,
Emest F Bizjak,
Eric L Dong, BS,
Eric M Mueller, PharmD,
Eric S Weilage,
Esther B Gamallo Herrera,
Esther C Broner, PhD,
Evelyn Wong,
Farhana Khan,
Felix Maldonado,
Frances Namuswe, PhD,
Frank W Perrella,
Freddy Ortiz Colon,
Frederick L Fricke,
Gabriel M Guevarra,
Gam S Zamil,
Gayle S Lawson,
Gene D Arcy,
Geraldine D Sanders,
Gerard Pde Leon,
German Rivera,
Gloria J Baca, MS,
Greg K Keshishyan,
Gregory A Berg,
Haley H Seymour,
Hanna L Potter,
Haroon Vohra (NMI),
Hasan A Irier, PhD,
Heidy C Perales,
Heika R Bounds,
Heika R Tait,
Himanshu Gupta, PhD,
Ian J Thomson,
Iraida Ortiz,
Iris C Macinnes,
Ivis L Negron,
Jacqueline Mdiaz Albertini,
James C Maclaughlin,
James D Hildreth,
James D Planchon,
James I Giefer,
James M Timper Jr,
James P Mcreavey,
James P Stumpff,
James R Fleckenstein,
Jamie M Du,
Jane S Wernberg,
Janet D Blount,
Jasmine N Thompson,
Javier O Vega,
Jee Chung, PhD,
Jeffery A Hangartner,
Jeffery A Johnson,
Jeffrey J Leclair,
Jeffrey P Raimondi,
Jennifer Gogley,
Jennifer L Bridgewater,
Jennifer L Huntington,
Jennifer M Gogley,
Jinnie Kokiatkulkij,
Joan T Briones,
Joanne E King,
Jocelyn E Massey,
Jocelyn E Sparks,
Joe X Phillips,
Joel Welch, PhD,
Joey V Quitania,
John A Gonzalez,
John Burr,
John D White,
John M Dietrick,
John R Myung,
Jonathan T Little,
Jorge E Martinez,
Jorge L Guadalupe,
Jose A Lopez,
Jose Acruz Gonzalez,
José E Meléndez,
Jose R Hernandez,
Jose Velez,
Joseph P Iser, MD,
Joseph X Phillips,
Joshua P Wireman,
Julia E Chervoni,
Julie A Rorberg,
Julie A Stocklin,
Junho Pak,
Ka L Wong,
Karen A Wolnik,
Karen Takahashi,
Kari M Johansen,
Katherine E Jacobitz,
Katherine Szestypalow,
Kathleen B Swat,
Kathleen R Jones, PhD,
Kathryn A Krentz,
Kathy Chiu,
Kathy Kuo,
Kelley,
Kelly I Anderson,
Kelvin Cheung,
Kelvin X Sanders,
Kendra A Biddick,
Kenneth H Williams,
Kenneth O Gee, PhD,
Kent C Faul,
Kevin A Gonzalez,
Kevin P Foley,
Kham Phommachanh,
Khoa Nathanv Tran,
Kim Lthomas Cruse,
Kimberley A Hoefen,
Kimberly M Lichter,
Kirtida Patel,
Kristin M Abaonza,
Kristy A Zielny,
Kumar G Janoria,
Lance Mde Souza, MBA,
Larry K Austin,
Laura Fontan, MS,
Laurel A Beer,
Lawrence J Stringer,
Lawrence Y Lee, PhD,
LCDR Chad N Thompson,
LCDR Debra Emerson,
Leonard H Lavi,
Liatte Kreuger, PharmD,
Liming Zhang,
Linda F Murphy,
Linda S Leja,
Linda Thai,
Ling Yu L Liu,
Lisa K Capron,
Lisa Shin,
Lori J Silverstein,
Louis B Cencetti,
Lourdes Andujar,
LT Richard A Lyght,
Luella J Rossi,
Luis A Dasta,
Luis Mburgos Medero,
Luis P Torres,
Lynda L Perry, PhD,
Marcelo O Mangalindan, Jr,
Marcia B Williams,
Marco S Esteves,
Marcus F Yambot,
Margaret M Annes,
Maria Pkelly Doggett, MBA,
Maribeth G Niesen,
Maridalia Torres Irizarry,
Marie Falcone,
Marie T Falcone,
Marijo B Kambere, PhD,
Marisol Faberlle,
Mariza M Jafary,
Mark A Tucker,
Mark C Saale,
Mark W Babbitt,
Marvin D Jones,
Matt D Suedkamp,
Matthew B Casale,
Matthew J Johnson,
Matthew J Sienko,
Maxine H Wong,
Maxyne T Lam,
Maya M Davis,
Megan A Haggerty,
Melissa D Ray,
Melissa J Garcia,
Michael A Charles,
Michael D Garcia,
Michael E Maselli,
Michael J Nerz,
Michael L Casner,
Michele L Obert,
Michelle A Marsh,
Miguel A Martinez Perez,
Mihaly S Ligmond,
Mikel T Wright,
Minh D Phan,
Mohsen Rajabi Abhari, FDA,
Moraima Jramos Valle,
Mra Davism,
Mra Greenc,
Mra Mcculloughj,
Mra Munizn,
Mra V Millarw,
Nabeela J Chaudhry,
Nancy A Saxenian Emmons,
Nancy G Schmidt,
Nancy L Rolli,
Nebil A Oumer,
Nelly N Tran,
Nicholas A Violand,
Nicholas L Hunt,
Nicholas L Paulin,
Nicholas Obiri, PhD,
Nicola M Fenty Stewart,
Nicole E Knowlton,
Nicole K Trudel,
Noreen Muñiz,
Omotunde O Osunsanmi,
Parul M Patel,
Patrick C Klotzbuecher,
Patrick L Wisor,
Patsy J Domingo,
Paul A Bonneau,
Paul F Rader,
Paul M Kawamoto,
Paul Mouris,
Paul Rader,
Paula A Trost,
Peter C Chow,
Peter S Diak,
Philip F Istafanos, DMV, MS,
Prabhu P Raju,
Prasad Peri, PhD,
Rachelle M Deskins,
Ralph A Erickson,
Randy L Self,
Raquel Gonzalez Rivera,
Raymond T Oji,
Rebecca Rodriguez,
Reyes Candau Chacon, PhD,
Richard C Chiang,
Richard Heath Coats,
Richard W Thornton,
Richmond K Yip,
Robert C Coleman,
Robert D Tollefsen,
Robert Darius,
Robert Jennings,
Roger F Zabinski,
Rona Leblanc, PhD,
Ronald G Crawford,
Ronald L Koller,
Ronda Leblanc,
Rowena S Nguyen,
Rumany C Penn, PharmD,
Ryan J Borges,
S Lori Brown, PhD MPH,
Saied A Asbagh,
Saleem A Akhtar,
Samantha J Bradley,
Samina S Khan,
Sandra L Shire, DMD, MPA,
Sangeeta M Khurana, PhD,
Sangeeta M Rataul,
Santiago Gallardo Johnson,
Santos E Camara,
Sara H Gabel,
Sarah A Hassas,
Sarah Arden,
Sarah E Mcmullen,
Scott R Nichols, PhD,
Sean P Desbrow,
Selene T Torres,
Shannon B Ruelle,
Sharon I Gundersen,
Shirley H Isbill,
Shirley J Berryman,
Sidney B Priesmeyer,
Sidonie J Takougang,
Simone E Pitts,
Sixto M Mercado Rios,
Sonia R Peterson,
Sonya L Karsik, RAC,
Sparky L Bartee,
Stanley Au,
Stephanie A Slater, MS,
Stephanie Mangigian, MS/OSH, RN,
Stephen D Brown,
Steven C Madzo,
Steven E Porter, Jr,
Steven Fong, MS, PhD,
Steven M Weinman,
Steven P Donald,
Sudipan Karmakar,
Sue Lee Chan,
Sundy V Sedwick,
Susan F Laska, MS,
Susan T Hadman,
Susan W Ting,
Susanna E Ford,
Taichun Qin, PhD,
Tamala P Bogan,
Tamala P Magee,
Tamika White,
Tara G Bizjak,
Tara R Gooen,
Temar Q Williams,
Teresa I Navas,
Thanh M Andrews,
Thao T Kwan,
Thao X Tran,
Thomas J Arista,
Thuy T Nguyen, LCDR,
Timothy D Evans,
Timothy T Kapsala,
Tina M Pawlowski,
Tina S Roecklein,
Torrance J Slayton,
Tracey T Duong,
Tracy K Li,
Truong Xuan Nguyen (Andy),
Uttaniti Limchumroon (Tom),
Vashti E Bocker,
Veronica Fuentes, MS,
Vickie L Anderson,
Vilmary Negron Rodriguez,
Virgilio F Pacio, CSO,
Viviana Matta,
Vlada Matusovsky,
Walden H Lee,
Wayne E Seifert,
Wayne T Smith,
William J Leonard,
Xiomara Copeland,
Yasamin Ameri,
Yumi J Hiramine,
Yvette Mlacour Davis,
Yvonne C Wilkes,
Yvonne T Lacour,
Zachary A Bogorad,
Zachary L Miller,
Zachary L Stamm
Terri L Dodds's Documents
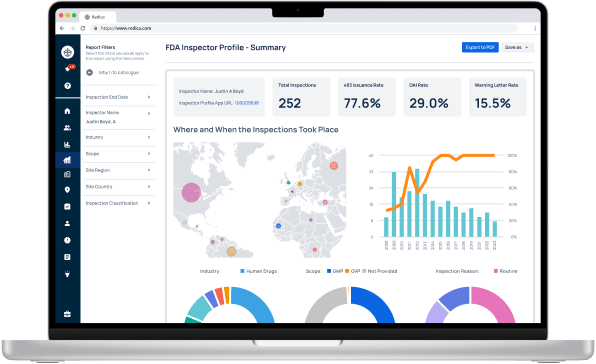
Experience Redica Systems’ NEW investigator profiles and dashboards
Redica Systems customers get access to over 1,400 FDA Investigator profiles, containing powerful analysis like:
- Total inspections conducted
- 483 rate
- OAI rate
- Warning Letter rate
- Inspections by Industry, Scope, and Inspection Reason
- And much more
Investigator Profiles are not sold as on-demand documents. If you’d like to see one, fill out the form below to connect with our Sales Team and get it in your inbox as little as 24 hours.
Talk to sales to access our investigator profiles
Loading...
Investigator Profiles Are Just the Tip of the Iceberg
Becoming a Redica Systems customer not only provides you with unlimited access to profiles similar to the one above for every current and former FDA Investigator, but also grants you access to:
Inspection Intelligence
- Pre-Approval Inspection (PAI) trends
- The ability to filter nearly any FDA enforcement action by GxP labels like GMP, GCP, etc.
- Latest 483s: see what the trends are across all FDA Investigators
- CFR Heatmap: Citations aggregated by Subpart by Year
Vendor Intelligence
- Full inspection histories for your vendors down to the specific site level
- All of the functionality mentioned above but isolated to just your vendors
Regulatory Intelligence
- Zero in on the trends for a specific agency/regulator (ex. FDA, MHRA, EMA, Health Canada)
- All the latest "Signals" from agencies around the world, segmented by Country, Type (ex. Guidance, Decree...), Category (ex. Rules/Regulations/Guidance, News, Reports...), Theme (ex. Cell and Gene Therapy, AI...), and more