FDA Investigator John D White
John D White has conducted inspections on 25 sites in 10 countries as of 25 Apr 2005. See below for a list of the FDA enforcement documents resulting from those inspections.
Investigator Details
Number of Inspected Sites:
25
Last Inspection Date:
25 Apr 2005
Investigator Role:
FDA Investigator
Redica ID:
Countries of Inspections:
France,
Ireland,
United States of America,
Germany,
Australia,
Canada,
China,
Japan,
United Kingdom of Great Britain and Northern Ireland,
Italy
FDA Investigators that have inspected at least one site in common with John D White:
Alan P Kurtzberg,
Alberto A Viciedo,
Althea A Williams,
Angela E Glenn,
Anh M Lac,
Anita Narula, PhD,
Ann L Demarco,
Anthony A Charity,
Arthur T Ogdahl,
Azza Talaat,
Barbara J Rincon,
Bijoy Panicker,
Bo Chi, PhD,
Bonita S Chester,
Brenda W Uratani, PhD,
Brooke K Higgins,
Candido Torres,
Carl A Huffman, III,
Carl Lee,
Carolyn E Barney,
CDR Rochelle B Young, RPh, MSA,
Charles M Edwards,
Charles R Cote, RIC,
Chiang Syin, PhD,
Christopher Downey, PhD,
Christopher S Keating,
Concepcion Cruz, Jr,
Constantin Y Philopoulos,
Cynthia J Lee, MS,
Dawn E Barkans,
Denise M Digiulio,
Dennis Cantellops Paite,
Devaughn Edwards,
Diane L Raccasi,
Doan T Nguyen, PharmD,
Donald C Obenhuber, PhD,
Donald L Lech,
Dr. Chunchang Fang,
Dr. Ralph M Bernstein, PhD,
Dr. Robert C Horan, MD,
Dr. Willie F Vann, PhD,
Dr. Zhihao Qiu (Peter), PhD,
Edecia A Richards,
Eliezar Ramos,
Elizabeth B Griffin,
Erika E Englund, PhD,
Erika V Butler,
Felix Maldonado,
Garry H Stewart,
George Pyramides,
Ivis L Negron,
James C Maclaughlin,
James I Giefer,
James R Birkenstamm,
James W Leonette,
James W Plucinski,
Jeffrey A Sommers,
Jeffrey D Meng,
Jennifer J Stone,
Jennifer L Huntington,
Jonathan W Chapman,
Jorge L Gonzalez,
Jorge L Guadalupe,
Jose A Lopez,
Jose R Hernandez,
Joyce A Kellett,
Julie D Bringger,
Justin A Boyd,
Kalavati Suvarna, PhD,
Kara D Dobbin,
Karyn M Campbell,
Katherine Szestypalow,
Kathleen R Jones, PhD,
Kevin A Gonzalez,
Kevin D Kallander,
Khoa Nathanv Tran,
Kristen D Evans,
Kurt A Brorson, PhD,
Larry K Austin,
Latorie S Jones,
LCDR Wilfred A Darang,
Loretta Nemchik,
Luella J Rossi,
Luis A Dasta,
Margaret M Annes,
Mariza M Jafary,
Mark Brunswick, PhD,
Mark E Parmon,
Mark P Prusak,
Mary E Storch,
Matthew B Casale,
Megan A Haggerty,
Michael A Charles,
Michael A Taylor,
Michael K Larson,
Michael L Chasey,
Michael R Goga,
Michael Shanks, MS,
Michelle D Haamid,
Michelle M Noe Varga,
Michelle Yclark Stuart,
Mihaly S Ligmond,
Milos Dokmanovic, PhD,
Monica Commerford, PhD,
Mra Munizn,
Nasir Ali, PhD,
Noreen Muñiz,
Omotunde O Osunsanmi,
Patrick C Klotzbuecher,
Paul M Kawamoto,
Peter E Baker,
Philip F Istafanos, DMV, MS,
Prabhu P Raju,
Qing Joanna Zhou, PhD,
Reba A Gates,
Regina T Brown,
Reyes Candau Chacon, PhD,
Richard E Needham,
Richard L Friedman,
Richard L Rutherford,
Richard Ledwidge (nmi), PhD,
Richard W Berning,
Robert D Tollefsen,
Robert J Martin,
Robert Jennings,
Rochelle K Kimmel,
Roger F Zabinski,
Rose Ashley,
Sarah E Mcmullen,
Scott T Ballard,
Sharon K Thoma, PharmD,
Simone E Pitts,
Stephen J Koniers,
Steven M Weinman,
Susan F Laska, MS,
Susan W Ting,
Syed N Ali,
Tamara Brey,
Tania K Mercado,
Temar Q Williams,
Teresa C Thompson,
Teresa I Navas,
Teresa Jimenez,
Terri L Dodds,
Thomas E Friel,
Thomas H Jenkins,
Thomas J Arista,
Thuy T Nguyen, LCDR,
Torrance J Slayton,
Veronica Fuentes, MS,
Victor Spanioli,
Virgilio F Pacio, CSO,
Walter N Lange,
Wayne D Mcgrath,
Wayne E Seifert,
Yanming An, PhD,
Yasamin Ameri,
Yvette I Johnson,
Zedong Dong,
Zerita White,
Zhong Li, PhD,
Ziyang Su, PhD
John D White's Documents
| Publish Date | Document Type | Title |
|---|---|---|
| September, 2002 | EIR | Boehringer Ingelheim Pharma GmbH and Co. KG - EIR, 2002-09-18 |
| April, 2003 | FDA 483 | KAKEN Pharmaceutical Co., LTD - Form 483, 2003-04-10 |
| September, 2002 | FDA 483 Response | Boehringer Ingelheim Pharma GmbH & Co. KG - Form 483R, 2002-11-27 |
| September, 2002 | FDA 483 Response | Boehringer Ingelheim Pharma GmbH and Co. KG - Form 483R, 2002-11-22 |
| April, 2003 | EIR | Kyowa Hakko Kirin Co., Ltd. - EIR, 2003-04-03 |
| September, 2003 | FDA 483 | PRIMED INSTRUMENTS INC - Form 483, 2003-09-17 |
| April, 2003 | FDA 483 Response | Kyowa Hakko Kirin Co., Ltd. - Form 483R, 2003-06-20 |
| September, 2002 | EIR | Boehringer Ingelheim Pharma GmbH & Co. KG - EIR, 2002-09-16 |
| May, 2001 | FDA 483 | Jiangsu Hengrui Medicine Co., Ltd. - Form 483, 2001-05-25 |
Experience Redica Systems’ NEW investigator profiles and dashboards
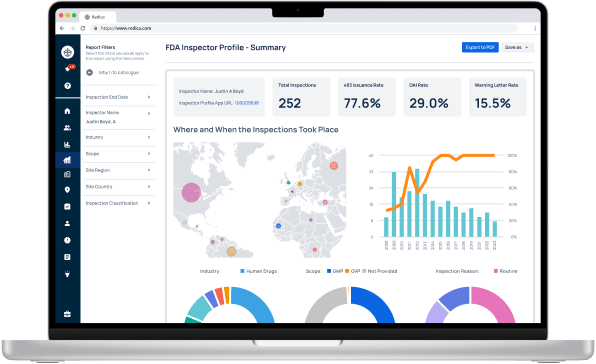
Redica Systems customers get access to over 1,400 FDA Investigator profiles, containing powerful analysis like:
- Total inspections conducted
- 483 rate
- OAI rate
- Warning Letter rate
- Inspections by Industry, Scope, and Inspection Reason
- And much more
Investigator Profiles are not sold as on-demand documents. If you’d like to see one, fill out the form below to connect with our Sales Team and get it in your inbox as little as 24 hours.
Talk to sales to access our investigator profiles
Loading...
Investigator Profiles Are Just the Tip of the Iceberg
Becoming a Redica Systems customer not only provides you with unlimited access to profiles similar to the one above for every current and former FDA Investigator, but also grants you access to:
Inspection Intelligence
- Pre-Approval Inspection (PAI) trends
- The ability to filter nearly any FDA enforcement action by GxP labels like GMP, GCP, etc.
- Latest 483s: see what the trends are across all FDA Investigators
- CFR Heatmap: Citations aggregated by Subpart by Year
Vendor Intelligence
- Full inspection histories for your vendors down to the specific site level
- All of the functionality mentioned above but isolated to just your vendors
Regulatory Intelligence
- Zero in on the trends for a specific agency/regulator (ex. FDA, MHRA, EMA, Health Canada)
- All the latest "Signals" from agencies around the world, segmented by Country, Type (ex. Guidance, Decree...), Category (ex. Rules/Regulations/Guidance, News, Reports...), Theme (ex. Cell and Gene Therapy, AI...), and more