FDA Investigator Scott T Ballard
Scott T Ballard has conducted inspections on 250 sites in 19 countries as of 27 Feb 2024. See below for a list of the FDA enforcement documents resulting from those inspections.
Investigator Details
Number of Inspected Sites:
250
Last Inspection Date:
27 Feb 2024
Investigator Role:
FDA Investigator
Redica ID:
Countries of Inspections:
United States of America,
India,
China,
Slovenia,
Taiwan,
Germany,
Belgium,
Spain,
Italy,
Denmark,
United Kingdom of Great Britain and Northern Ireland,
Puerto Rico,
France,
Japan,
Ireland,
Australia,
Dominican Republic,
Sweden,
Netherlands
FDA Investigators that have inspected at least one site in common with Scott T Ballard:
Adaliz Santaliz Cruz,
Adam C Hipko,
Adaris Mas Rivera,
Ademola O Daramola,
Adetutu M Gidado,
Aditi S Thakur,
Adrian Rodriguez,
Akbar J Zaidi,
Alan L Truong,
Alan P Kurtzberg,
Alanna L Mussawwir Bias,
Albert F Peacock, PhD,
Alcee M Tavarez,
Alexandra B Pitkin,
Alice C Silva,
Alicia M Mozzachio,
Amanda J White,
Anastasia M Shields,
Andrea A Branche,
Andrea J Schmidt,
Andrew A Hoopes,
Andrew Byrnes, PhD,
Andrew J Barrowcliff,
Andrew J Brown,
Andrew J Lang,
Andrey Sarafanov, PhD,
Angel K Sandhu,
Angela E Glenn,
Angela Mock,
Anh M Lac,
Anita Narula, PhD,
Anitha Palamakula Govada,
Ann L Demarco,
Ann Marie Montemurro,
Anna Lazar,
Anna R Kwilas,
Annette Melendez,
Anthony F Lorenzo,
Anya Dlockett Evans,
April L Young,
Aqualia L Nelson,
Arie C Menachem,
Arie R Dyk,
Ariel Cruz Figueroa,
Arsen Karapetyan,
Ashar P Parikh,
Ashley A Mutawakkil,
Ashley N Queen, PhD,
Ashley N Williams,
Atul J Agrawal,
Ava M Bowman,
Azza Talaat,
Barbara Janine Breithaupt,
Barbara M Frazier,
Benjamin J Smith,
Bernardino E Mendez,
Bing Lin,
Binh T Nguyen,
Blondell Hill,
Blondell W Johnson,
Bo Chi, PhD,
Bobbi J Miller,
Bonita S Chester,
Bonnie L Menzel,
Brackens Harris,
Brandi E Williams,
Brandon C Heitmeier,
Brandon K Barbee,
Brandy N Lepage,
Brentley S Collins,
Brian D Nicholson,
Brian R Yaun,
Brian Sanders,
Burnell M Henry,
Byungja E Marciante,
Camerson E Moore,
Camille D Brown,
Carla A Norris,
Carla J Lundi,
Carlos Chavez,
Carol A Declouette Wilburn,
Cary Greene,
Caryn M Mcnab,
Cassandra L Abellard,
Cassandra L Winters,
Catherine J Laufmann,
Cathleen A Castellaw,
CDR Donald Ertel,
CDR Ileana Barreto Pettit,
CDR Jeremy L Wally, PhD,
CDR Rochelle B Young, RPh, MSA,
CDR Thomas R Berry, PPh,
Chad R Burger,
Charles D Brown,
Charles I Ann,
Charles M Edwards,
Charles M Spyr,
Charles R Cote, RIC,
Cheneysc,
Cheng H Yen,
Cheryl A Clausen,
Christian D Lynch (CDL),
Christie A Soto,
Christine E Kelley,
Christine I Shaw,
Christine W Twohy,
Christopher D Leach,
Christopher D Rush,
Christopher J Adams,
Christopher J Hurst,
Christopher J Smith,
Christopher L Jacobs,
Christopher N Tran,
Christopher R Czajka,
Christopher S Keating,
Christopher T Middendorf,
Christos G Tsingelis,
Chryste D Best,
Claire M Minden,
Clinton J Lott,
Cody D Rickman,
Connie L Hannon Rabel,
Constantin Y Philopoulos,
Courtney A Gilbert,
Creighton T Tuzon,
Crystal S Harris,
Cymbre Weatherly,
Cynthia A Harris, MD, RN,
Cynthia J Lee, MS,
Cynthia Jim, CSO,
Cynthia M Goudeau,
Cynthia R Gibson,
Dallas E Gilbreath Jr,
Damaris Y Hernandez,
Dana M Lewis,
Dandan Wang, PhD,
Daniel J Lahar,
Daniel J Roberts,
Daniel S Fam,
Danielle Lyke,
Danny D Horner,
Darla J Christopher,
Darren S Brown,
David A Paterson,
David Castillo,
David M Beltran,
Dawn C Olenjack,
Deborah M Trout,
Decarlos A Gomez,
Dell S Moller,
Demario L Walls,
Denise Connelly,
Denise M Digiulio,
Deniza Karacic,
Dennis Cantellops Paite,
Destry M Sillivan,
Devaughn Edwards,
Diana L Ayala,
Diane L Raccasi,
Dipesh K Shah,
Djamila Harouaka,
Doan N Singh,
Doan T Nguyen, PharmD,
Don H Bark, PhD,
Donald L Lech,
Dongping Dai, PhD,
Douglas A Campbell,
Douglas C Kovacs,
Dr. Barbara D Paul, PhD,
Dr. Chunchang Fang,
Dr. Gopa Biswas, PhD,
Dr. Jason R Caballero,
Dr. Jun Liu, PhD,
Dr. Ralph M Bernstein, PhD,
Dr. Robert C Horan, MD,
Dr. Zhihao Qiu (Peter), PhD,
Dusty F Snoeberg,
Edmiston,
Edmund F Mrak, Jr,
Edward D Edmiston,
Edward Deberry,
Edwin Melendez,
Edwin Ramos,
Eileen A Liu,
Eileen J Bannerman,
Elisa M Fleming,
Elizabeth D Connell,
Elizabeth Ll Edwards,
Ellen P Madigan,
Elmina E Akwo,
Elmina E Mboh,
Elvia J Cervantes,
Emest F Bizjak,
Emilio O Escobar,
Emily B Camire,
Emily J Orban,
Emine Guvenmaiorov,
Emmanuel Adu Gyamfi, PhD,
Enrico Joset Mangahis,
Eric L Dong, BS,
Eric M Mueller, PharmD,
Erick G Marchan,
Erika V Butler,
Erin D Mccaffery,
Erma Zaimova,
Ernest Serna, III,
Esther A Ofori,
Everard A Irish,
Ewa Marszal, PhD,
Fabian Nchaparro Rodriguez,
Farhana Khan,
Felix Maldonado,
Fernando C Martinez,
Florecia S Philogene,
Frances L Dejesus,
Francis A Guidry,
Freddy Ortiz Colon,
Frederic W French, III,
Gabriel M Guevarra,
Gabriel R Mclemore,
Gam S Zamil,
Garrad R Poole,
Gene D Arcy,
George J Flynn,
German Rivera,
Gery M Duparc,
Ginger M Sykes,
Glasgow Glasgow,
Graeme E Price,
Graeme Price,
Grant L Davis,
Gregory A Holt,
Gregory W Smith,
Gregson A Joseph,
Guo Q Shen,
Gwyn G Dickinson,
H Ljamilla Selby,
Haijing Hu,
Hala L Selby,
Harry J Brewer,
Hasan A Irier, PhD,
Helen B Ricalde,
Holly M Scott,
Howard Anderson, PhD,
Hung V Le,
Ifueko Osemwota,
Iris C Macinnes,
Ivan E Reyes,
Ivis L Negron,
J David Doleski,
J Elkins,
Jacey Roy,
Jacqueline Mdiaz Albertini,
Jakob Reiser, PhD,
James C Maclaughlin,
James L Dunnie, Jr,
James P Stumpff,
James S Cartwright,
Jamie M Bumpas,
Jana L Caylor,
Jane L Chang,
Jane M Broussard,
Janet B Abt,
Janice M Hickok,
Jared P Stevens,
Jason F Chancey,
Jason P Aun,
Jd Young,
Jeffery A Hangartner,
Jeffrey A Sommers,
Jeffrey D Meng,
Jeffrey P Raimondi,
Jeffrey R Wooley,
Jennifer D Young,
Jennifer Hua,
Jennifer L Huntington,
Jennifer L Reed,
Jennifer L Schmidt,
Jennifer Lalama,
Jennifer O Dowdy,
Jenny Agila Sefen,
Jessica L Pressley,
Jie He,
Jill J Tillman,
Joan A Loreng,
Joanne E King,
Joanne M Schlossin,
Jodi M Gatica,
Joel D Martinez,
Joey V Quitania,
Johann M Fitch,
John A Gonzalez,
John A Iwen,
John D White,
John T Chapman,
Johnson,
Jolanna A Norton,
Jonah S Ufferfilge,
Jonaida Estevez Martinez,
Jonathan A Womack,
Jonathan G Matrisciano,
Jonathan M Mcbride,
Jonathan W Chapman,
Jorge L Guadalupe,
Jose A Lopez,
Jose A Moreno,
Jose Acruz Gonzalez,
José E Meléndez,
Jose M Cayuela,
Jose Martinez, Jr,
Jose R Hernandez,
Joseph E Duran,
Joseph George,
Joseph R Lambert,
Joshua L Gibbs,
Juan D Lopez,
Juanita P Versace,
Julia E Chervoni,
June P Page,
Junho Pak,
Justin A Boyd,
Kalavati Suvarna, PhD,
Karen C Daugherty,
Kari M Johansen,
Karl D Hezel,
Karlton T Watson,
Karyn M Campbell,
Katherine E Jacobitz,
Katherine Szestypalow,
Kathleen Chmura Shaffer,
Kayla V Sprague,
Kejun Cheng,
Kelley,
Kellia N Hicks,
Kelly D Moore,
Kenitra D Hewitt,
Kenneth H Williams,
Kent C Faul,
Kevin A Gonzalez,
Kevin P Foley,
Kevin T Nguyen,
Kham Phommachanh,
Khunnathee Stoner,
Kimberley A Hoefen,
Kimberly Cdelk Brooks,
Kip J Hanks,
Kristin N Longfellow,
Kunapuli T Madhusudhan,
L Grant,
Laiza V Garcia,
Lance Sindo, CIH, CQA, RS,
Larry K Austin,
Larry K Hampton,
Latorie S Jones,
Laura E Garcia,
Laura Fontan, MS,
Laureen M Geniusz,
Laurel A Beer,
Lauren E Skokan,
Lauren M Lilly, PhD,
Lauren R Brady,
Lauren R Howell,
Laurimer Kuilan Torres,
Laurissa S Flowers,
LCDR Debra Emerson,
LCDR Ismael Olvera, IV,
LCDR Michael H Tollon,
Leighton Kn Gai,
Lesley K Satterwhite,
Leslie W Gilbert,
Lewis K Antwi,
Libia M Lugo,
Lillian C Hsu,
Lillian Mcolon Mcknight,
Lillie M Young,
Liming Zhang,
Linda F Murphy,
Linda Thai,
Lindsey Brown, PhD,
Liqun Zhao,
Lisa L Flores,
Lisa M Puttonen,
Lisa R Jennings,
Lisa R Whitt,
Liza L Quiles,
Lloyd D Payne,
Lori B Wikstrom,
Lori G Cantin,
Lori Peters,
Lorie S Hannappel,
Lourdes Andujar,
Lourdes R Genera,
Lucas B Leake,
Lucila B Nwatu,
Luella J Rossi,
Luis A Dasta,
Luis Soto Lopez,
Lydia I Rosas Marty,
Madushini Dharmasena, PhD,
Maira P Brading,
Makini Cobourne Duval, PhD,
Malgorzata G Norton, PhD,
Marc A Jackson, Jr,
Marc R Dickens,
Marcellinus D Dordunoo,
Marcia G Evans,
Marcus F Yambot,
Margaret M Annes,
Maria Estrella,
Maria V Price,
Marie A Fadden,
Marie B Buen Bigornia,
Marie F Morin,
Marijo B Kambere, PhD,
Mario Seminara,
Marion Michaelis,
Marion W Nadeau,
Mariza M Jafary,
Mark A Guerra,
Mark E Parmon,
Mark I Schwartz,
Marla A Cassidy,
Marlene G Swider,
Martha S Baldwin,
Martrice A Packer,
Marvin D Jones,
Mary E Farbman, PhD,
Massoud Motamed,
Mathew T Thomas, MD,
Matthew A Johnson,
Matthew B Casale,
Matthew J Gretkierewicz,
Matthew L Doyle,
Matthew R Maddox,
Matthew R Noonan,
Megan A Haggerty,
Megan T Ziegler,
Meisha R Sampson,
Meisha Waters,
Melissa D Ray,
Melissa I Michurski,
Merelynn Rhoten,
Michael A Charles,
Michael C Kennedy, PhD,
Michael Havert, PhD,
Michael J Vardon,
Michael P Sheehan,
Michael R Goga,
Michael R Klapal,
Michael Shanks, MS,
Michael W Burd,
Michele L Forster, PhD,
Michele L Glendenning,
Michele L Obert,
Michelle A Krayer,
Michelle L Jones,
Michelle Paxon,
Michelle S Dunaway,
Miguel A Martinez Perez,
Mihaly S Ligmond,
Mikhail V Ovanesov, PhD,
Mindy M Chou,
Minh D Phan,
Mizanne E Lewis,
Monica Cburgos Garcia,
Monica J Wilkins,
Monika Borkowska,
Monique Rac Lester,
Monique S Frazier,
Mra Davism,
Mra M Barbosar,
Mra Mcculloughj,
Mra Munizn,
Muralidhara B Gavini, PhD,
Nadeem I Chaudhry,
Nadine Nanko Johnson,
Nancy G Schmidt,
Nancy Kirschbaum, PhD,
Nancy T Waites,
Nasir Ali, PhD,
Navista C Bolton,
Nawab A Siddiqui,
Nebil A Oumer,
Nicholas A Violand,
Nicole A Lloyd,
Nicole E Knowlton,
Nikki S Ramirez,
Nimmy Mathews,
Ninfa N Innocent,
Nirjal Bhattarai,
Nobuko H Katagiri,
Noreen Muñiz,
Olga Simakova, PhD,
Olumide A Martins,
Oluwasefunm Agbanigo,
Omotunde O Osunsanmi,
Pablo Feliciano,
Pal S Mayasandra,
Pankaj H Amin,
Pankaj K Mandal,
Parul M Patel,
Patrice S Hall,
Patricia M Jacobs,
Patrick C Klotzbuecher,
Patrick D Stone, MS,
Patsy J Domingo,
Patty P Kaewussdangkul,
Paul A Bonneau,
Paul E Frazier,
Paul E Stein,
Paul M Chefor,
Paul Mouris,
Paula A Trost,
Paula J Bretz,
Paula J Perry,
Pauline N Logan,
Payne,
Peng Zhou,
Penny H Mccarver,
Peter E Baker,
Philip M Steele, PhD,
Phillip C Thai,
Phillip J Sample,
Phillip M Pontikos,
Prabhu P Raju,
Pratik S Upadhyay, DDC,
Priscilla M Pastrana,
Qin Xu,
Qing Delgado,
R Edwadebey Deberry,
Rabia Ballica, PhD,
Rachel C Harrington,
Rachel C Stanton,
Ralph A Erickson,
Ramon A Hernandez,
Ramon E Martinez,
Randa Melhem, PhD,
Randy L Clarida,
Raymond W Brullo,
Rebecca Co Bryan,
Rebecca E Dombrowski,
Rebecca K Olin,
Rebecca Parrilla,
Rebecca Rodriguez,
Regina T Brown,
Rendall E Barfoot,
Rene R Ramirez,
Rhonda L Dash,
Rian L Pope,
Richard E Needham,
Richard H Penta,
Richard Heath Coats,
Richard Ledwidge (nmi), PhD,
Richard T Riggie,
Rita K Kabaso,
Robert C Coleman,
Robert C Steyert,
Robert D Tollefsen,
Robert J Ham,
Robert J Martin,
Robert Jennings,
Robert M Barbosa,
Robert M Tillman,
Robert T Harris,
Robert T Lorenz,
Rochelle K Kimmel,
Rochelle L Cross,
Rodney D Combs,
Rodney L Tinzie,
Roger F Zabinski,
Ronda Rloyd Jones,
Ronnie E Jackson,
Rose Ashley,
Rose Xu,
Ross J Grigsby,
Rowena Trevino Solis,
Roy Baby,
Roy C Stephens,
Roy Hendley,
Rumany C Penn, PharmD,
Rupa Pradhan,
Russ E Davis,
Russell J Glapion,
Russell K Riley,
Saied A Asbagh,
Saijal P Naik,
Sakineh Walther,
Saleem A Akhtar,
Salvatore N Randazzo,
Samina S Khan,
Samuel A Hamblin,
Sandra A Hughes,
Sandy J Ziegler,
Sangeeta M Khurana, PhD,
Santos E Camara,
Sara Rothman,
Sarah B Tanksley,
Sarah Ibrahim,
Sarah J Cullen,
Sarah M Meng,
Sarah M Napier,
Satheesh Thomas,
Scott B Laufenberg,
Sean C Johnson,
Sean M Cheney,
Sean P Desbrow,
Seneca D Toms,
Seri L Essary,
Sha'tina R Alridge,
Shafiq S Ahadi,
Shanna N Purdy,
Shannon A Gregory,
Shannon A Lowe,
Sharon K Thoma, PharmD,
Shavon L Square,
Shawn D Goldman,
Shawn E Larson,
Shelby N Turner,
Sherrie L Krolczyk,
Shirley J Berryman,
Sidney B Priesmeyer,
Simone E Pitts,
Sixto M Mercado Rios,
Sonia R Peterson,
Sonya L Karsik, RAC,
Sonya M Edmonds,
Stacey F Allard,
Stacey F Rivette,
Staci G Mcallister,
Stephanie A Slater, MS,
Stephanie D Crockett,
Stephen D Brown,
Stephen L Beekman,
Steven A Brettler,
Steven A Gonzales,
Steven D Dittert,
Steven D Kehoe,
Steven E Bowen, PhD,
Steven M Weinman,
Steven P Donald,
Stuart W Russell,
Sukyoung Sohn,
Sumit Sen,
Sunitha K Rajaram, PhD,
Susan F Laska, MS,
Susan M Jackson,
Susan M Turcovski,
Susan P Bruederle,
Susan T Hadman,
Susan W Ting,
Susanna E Ford,
Susanne M Richardson, MS RAC,
Suzanne N Vallez,
Syed N Ali,
Taichun Qin, PhD,
Tamala P Bogan,
Tamala P Magee,
Tamera H Hunt,
Tamil Arasu, PhD,
Tania Y Hall,
Tara A Gray,
Ted L Anderson,
Teresa Jimenez,
Terrance L Thomas,
Terri E Gibson,
Thai D Truong,
Thomas J Arista,
Thomas Martinez,
Thomas Siebertz,
Thuy T Nguyen, LCDR,
Tiara Nbrown Crosen,
Timothy B Webb,
Toby H Hill,
Toby Vern H Hill,
Tonia F Bernard,
Torrance J Slayton,
Tracy J Washington,
Travis M Beard,
Travis S Bradley,
Travis S Brown,
Tricia S Martinez,
Trina K Vick,
Truong Xuan Nguyen (Andy),
Unnee Ranjan,
Veronica Fuentes, MS,
Vijaya L Simhadri,
Vilmary Negron Rodriguez,
Virginia L Meeks,
Viviana Matta,
Vlada Matusovsky,
Walden H Lee,
Warren J Lopicka,
Wayne D Mcgrath,
Wayne E Seifert,
Wei Wang, PhD,
Wen Ning Chan (Sally),
William F Lagud, Jr,
Xiaohui Shen,
Xiaokuang Lai, PhD,
Xiaoping Guan,
Yasamin Ameri,
Yaw O Osei,
Yiwei Li,
Yonggang Wang, PhD,
Young M Yoon,
Yumi J Hiramine,
Yvins Dezan,
Yvonne E Lozano,
Zachary L Miller,
Ze Peng,
Zerita White,
Zhaoyang Meng,
Zhong Li, PhD,
Zonglin Hu,
Zumera B Ajani
Scott T Ballard's Documents
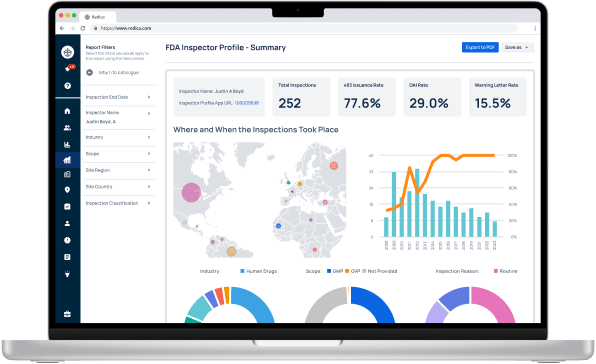
Experience Redica Systems’ NEW investigator profiles and dashboards
Redica Systems customers get access to over 1,400 FDA Investigator profiles, containing powerful analysis like:
- Total inspections conducted
- 483 rate
- OAI rate
- Warning Letter rate
- Inspections by Industry, Scope, and Inspection Reason
- And much more
Investigator Profiles are not sold as on-demand documents. If you’d like to see one, fill out the form below to connect with our Sales Team and get it in your inbox as little as 24 hours.
Talk to sales to access our investigator profiles
Loading...
Investigator Profiles Are Just the Tip of the Iceberg
Becoming a Redica Systems customer not only provides you with unlimited access to profiles similar to the one above for every current and former FDA Investigator, but also grants you access to:
Inspection Intelligence
- Pre-Approval Inspection (PAI) trends
- The ability to filter nearly any FDA enforcement action by GxP labels like GMP, GCP, etc.
- Latest 483s: see what the trends are across all FDA Investigators
- CFR Heatmap: Citations aggregated by Subpart by Year
Vendor Intelligence
- Full inspection histories for your vendors down to the specific site level
- All of the functionality mentioned above but isolated to just your vendors
Regulatory Intelligence
- Zero in on the trends for a specific agency/regulator (ex. FDA, MHRA, EMA, Health Canada)
- All the latest "Signals" from agencies around the world, segmented by Country, Type (ex. Guidance, Decree...), Category (ex. Rules/Regulations/Guidance, News, Reports...), Theme (ex. Cell and Gene Therapy, AI...), and more