FDA Investigator Scott N Lim
Scott N Lim has conducted inspections on 57 sites in 7 countries as of 20 Jun 2024. See below for a list of the FDA enforcement documents resulting from those inspections.
Investigator Details
Number of Inspected Sites:
57
Last Inspection Date:
20 Jun 2024
Investigator Role:
FDA Investigator
Redica ID:
Countries of Inspections:
United States of America,
India,
Canada,
United Kingdom of Great Britain and Northern Ireland,
Korea (Republic of),
Bulgaria,
China
FDA Investigators that have inspected at least one site in common with Scott N Lim:
Alan L Truong,
Alan P Kurtzberg,
Alanna L Mussawwir Bias,
Alex G Kalugin,
Alicia Aborg Borm,
Amir Alavi,
Andrea A Branche,
Andrea George, PhD,
Angel K Sandhu,
Angela E Glenn,
Anh M Lac,
Anita Narula, PhD,
Arindam Dasgupta, PhD,
Arsen Karapetyan,
Ashar P Parikh,
Atul J Agrawal,
Barbara J Rincon,
Barbara J White,
Barbara J Wilcox, PhD,
Binh T Nguyen,
Bo Chi, PhD,
Calvin B Koerner,
Carl Lee,
Carla J Lundi,
Carole L Jones,
Carolyn A Renshaw,
Carrie A Hughes,
Caryn M Mcnab,
Cathleen A Carr Sharpe,
CDR Ileana Barreto Pettit,
CDR Rochelle B Young, RPh, MSA,
Cecilia H Kieu,
Celena Ngo,
Cheryl A Clausen,
Chiang Syin, PhD,
Christopher D Leach,
Christopher M Jenner,
Christopher R Czajka,
Christopher S Keating,
Christopher T Middendorf,
Claudia Mperez Kasmarski,
Clinton J Lott,
Concepcion Cruz, Jr,
Connie P Rezendes,
Cynthia J Tsui,
Daniel J Lahar,
Daniel J Roberts,
David G Eng,
David J Gomes,
Deborah A Greco,
Diana L Ayala,
Diane Cvan Leeuwen,
Dipesh K Shah,
Donald B Mckechnie,
Dr. Mark J Seaton, PhD,
Dr. Ralph M Bernstein, PhD,
Dustin M James,
Dustin P Tran,
Dustin R Abaonza,
Edith M Gonzalez,
Emily Shacter,
Eric L Dong, BS,
Eric W Anderson,
Frances Namuswe, PhD,
Gerald Feldman,
Gerald M Feldman, PhD,
Hao Kiet D Phan,
Haoheng Yan, PhD,
Henry K Lau,
Humberto Z Gomez,
Hyung Yul Lee,
Isabel Y Espinosa,
Jacek Cieslak, PhD,
Jacob W Reynolds,
Jai P Singh,
James C Lee, PharmD,
James R Evans,
Jannifer L Degroot,
Jeanne Fringer, PhD,
Jeffrey M Watson,
Jeffrey P Raimondi,
Jennifer Crumb,
Jennifer L Degroot,
Jennifer L Johnson,
Jennifer Swisher, PhD,
Joan T Briones,
Joel Welch, PhD,
Joey V Quitania,
John A Gonzalez,
Jose A Lopez,
Jose Acruz Gonzalez,
Joseph Kutze, PhD,
Joseph P Iser, MD,
Joseph R Lambert,
Joshua P Wireman,
Joshua S Hunt,
Juan Rjimenez Garcia,
June P Page,
Junho Pak,
Justin A Boyd,
Kathryn E King,
Keith O Webber, PhD,
Keith Webber, PhD,
Kelley,
Kellia N Hicks,
Kenneth S Boehnen,
Kent A Conforti,
Kevin P Foley,
Kham Phommachanh,
Khoa Nathanv Tran,
Kim Lthomas Cruse,
Kouros Kangarli,
Kristin M Abaonza,
Kurt A Brorson, PhD,
Lakisha M Williams,
Lam,
Lance Mde Souza, MBA,
Lance W Bohm,
Laurie Graham,
Laurie P Norwood,
LCDR Jennifer H Rhyu,
Liming Zhang,
Linan Ha, PhD,
Linda F Murphy,
Linh Tu,
Lisa Shin,
Lucila B Nwatu,
Mabel M Lee,
Maida Henesian (NMI),
Marcus A Goshen,
Marie K Kinkade,
Mariza M Jafary,
Marsha W Major,
Marshalette O Edwards,
Mary Ewilkerson Brinsko,
Matthew A Johnson,
Matthew B Casale,
Mei Chiung Huang (Jo),
Michael D Garcia,
Michael R Goga,
Michael Shanks, MS,
Michael T Cyrus,
Michelle Rfrazier Jessen, PhD,
Mihaly S Ligmond,
Mikhail V Ovanesov, PhD,
Milos Dokmanovic, PhD,
Min Shanmabel Liu,
Muralidhara B Gavini, PhD,
Nabeela J Chaudhry,
Nadeem I Chaudhry,
Nancy E Byerly,
Natalia Pripuzova, PhD,
Nayan J Patel,
Nicholas L Hunt,
Nicholas Obiri, PhD,
Omotunde O Osunsanmi,
Patricia F Hughes, PhD,
Patrick C Klotzbuecher,
Peter Adams, PhD,
Peter E Baker,
Peter Kessler, PhD,
Qing Joanna Zhou, PhD,
Raymond T Oji,
Reba A Gates,
Rebecca Rodriguez,
Rebecca T Davis,
Reyes Candau Chacon, PhD,
Richard Ledwidge (nmi), PhD,
Riley C Myers, PhD,
Robert D Tollefsen,
Roger F Zabinski,
Rumany C Penn, PharmD,
Ruth Moore, PhD,
Saied A Asbagh,
Saleem A Akhtar,
Sammie P La,
Sandra S Saniga,
Sangeeta M Khurana, PhD,
Santiago Gallardo Johnson,
Seneca D Toms,
Serge L Beaucage,
Sharon K Thoma, PharmD,
Sheilyn H Huang,
Shelby N Turner,
Shelley H Beausoleil,
Sherri J Jackson,
Sherrie M La,
Sidney B Priesmeyer,
Sonia R Peterson,
Sparky L Bartee,
Sri Ramakrishnaiah Yellela, PhD,
Stephanie A Slater, MS,
Stephanie Barragan,
Stuart W Russell,
Terrence Gee,
Thao T Kwan,
Thea C Grome,
Theresa Kirkham (NMI),
Thomas J Arista,
Thomas W Gordon,
Thuy T Nguyen, LCDR,
Tiara Nbrown Crosen,
Timothy C Grome,
Timothy T Kapsala,
Tomika L Bivens,
Tracy Denison, PhD,
Truong Xuan Nguyen (Andy),
Uttaniti Limchumroon (Tom),
Vaishali J Patel,
Vashti E Bocker,
Victoria L Palmer,
Virgilio F Pacio, CSO,
Viviana Matta,
Walden H Lee,
Wayne E Seifert,
William A Burkey,
William J Leonard,
William V Millar,
Yen Tso Kuo,
Yi Wang, PhD,
Yumi J Hiramine,
Zachary A Bogorad,
Zachary L Stamm,
Zhong Li, PhD
Scott N Lim's Documents
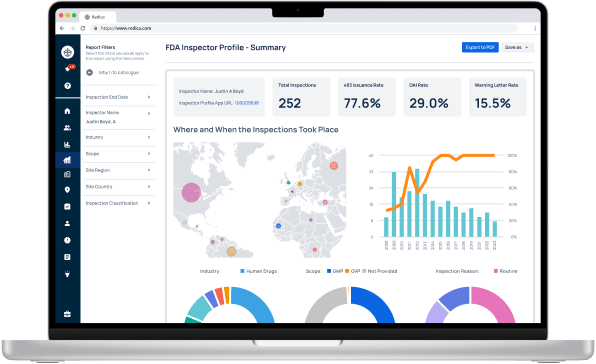
Experience Redica Systems’ NEW investigator profiles and dashboards
Redica Systems customers get access to over 1,400 FDA Investigator profiles, containing powerful analysis like:
- Total inspections conducted
- 483 rate
- OAI rate
- Warning Letter rate
- Inspections by Industry, Scope, and Inspection Reason
- And much more
Investigator Profiles are not sold as on-demand documents. If you’d like to see one, fill out the form below to connect with our Sales Team and get it in your inbox as little as 24 hours.
Talk to sales to access our investigator profiles
Loading...
Investigator Profiles Are Just the Tip of the Iceberg
Becoming a Redica Systems customer not only provides you with unlimited access to profiles similar to the one above for every current and former FDA Investigator, but also grants you access to:
Inspection Intelligence
- Pre-Approval Inspection (PAI) trends
- The ability to filter nearly any FDA enforcement action by GxP labels like GMP, GCP, etc.
- Latest 483s: see what the trends are across all FDA Investigators
- CFR Heatmap: Citations aggregated by Subpart by Year
Vendor Intelligence
- Full inspection histories for your vendors down to the specific site level
- All of the functionality mentioned above but isolated to just your vendors
Regulatory Intelligence
- Zero in on the trends for a specific agency/regulator (ex. FDA, MHRA, EMA, Health Canada)
- All the latest "Signals" from agencies around the world, segmented by Country, Type (ex. Guidance, Decree...), Category (ex. Rules/Regulations/Guidance, News, Reports...), Theme (ex. Cell and Gene Therapy, AI...), and more