FDA Investigator S Lori Brown, PhD MPH
S Lori Brown, PhD MPH has conducted inspections on 149 sites in 19 countries as of 18 Oct 2013. See below for a list of the FDA enforcement documents resulting from those inspections.
Investigator Details
Number of Inspected Sites:
149
Last Inspection Date:
18 Oct 2013
Investigator Role:
FDA Investigation Participant
Redica ID:
Countries of Inspections:
United States of America,
Japan,
India,
Finland,
China,
Romania,
Germany,
France,
Italy,
Norway,
Poland,
Mexico,
Greece,
South Africa,
Canada,
Hong Kong,
Ireland,
Czechia,
United Kingdom of Great Britain and Northern Ireland
FDA Investigators that have inspected at least one site in common with S Lori Brown, PhD MPH:
Aaron J Poloni,
Abby M Miller,
Ademola O Daramola,
Alan D Grupe,
Alan P Kurtzberg,
Alexander M Kay,
Alexandra B Pitkin,
Alice S Tsao,
Amy R Glynn,
Ana P Pineda Zavaleta,
Anastasia M Shields,
Andrea A Branche,
Andrew "Drew" Haack, PhD,
Andrew K Haack, PhD,
Andrew Le,
Andrew S Johnson,
Angela E Glenn,
Anh Trinh T Nguyen,
Anissa M Vargas,
Anita Narula, PhD,
Ann L Demarco,
Anthony R Petriella,
Arie C Menachem,
Arsen Karapetyan,
Ashar P Parikh,
Astrida B Mattson,
Aurora Miha Trifanov,
Avery J Dennis,
Azza Talaat,
Barbara D Wright,
Barbara I Rogolsky,
Barbara Janine Breithaupt,
Barbara M Frazier,
Bijoy Panicker,
Binh T Nguyen,
Bradley Dworak, PhD,
Brandy N Lepage,
Brenda K Alford,
Brenda L Reihing,
Brian D Nicholson,
Brian L Belz,
Brian M Janelsins, PhD,
Brian P Hendrickson,
Bruce R Burrell,
Bryan L Mcguckin,
Bryce E Mansfield,
Burnell M Henry,
Camille Bennett Hoffman,
Carl A Anderson,
Carla J Lundi,
Caryn M Everly,
Caryn M Mcnab,
Catherine J Laufmann,
CDR Donald Ertel,
CDR Ileana Barreto Pettit,
CDR Rochelle B Young, RPh, MSA,
Celia L Hutchinstein,
Charisse K Green,
Charles M Edwards,
Charles Yuanchia Kuo, PhD,
Chava Kimchi Sarfaty, PhD,
Cheryl A Clausen,
Christian D Lynch (CDL),
Christina V Santos,
Christine A Harman, PhD,
Christopher R Czajka,
Christopher S Genther,
Christopher S Keating,
Colleen F Hoyt,
Concepcion Cruz, Jr,
Connie P Rezendes,
Courtney N Long,
Crystal A Harlan,
Ct Viswanathan, PhD,
Cynthia J Lee, MS,
Cynthia L Gorveatt,
Dan C Fisher,
Darrin E Davis,
David J Gomes,
David L Hunsaker,
Dawn E Barkans,
Deborah A Nebenzahl,
Deborah M Trout,
Debra D Devlieger,
Dennis Cantellops Paite,
Dennis G Kawabata,
Devaughn Edwards,
Devon M Shoop,
Diane L Raccasi,
Dipesh K Shah,
Dirk L Lincoln,
Djamila Harouaka,
Doan T Nguyen, PharmD,
Dolores E Price,
Don H Bark, PhD,
Donald B Mckechnie,
Dov H Pluznik, PhD,
Dr. Barbara D Paul, PhD,
Dr. Guang Gao, PhD,
Dr. Ralph M Bernstein, PhD,
Edwin Melendez,
Eileen A Liu,
Elizabeth A Sheller,
Elizabeth S Howell,
Eric L Scott,
Eric M Mueller, PharmD,
Erika V Butler,
Felix Maldonado,
Francis A Guidry,
Gavin W Pierce,
Gene D Arcy,
George Pyramides,
Gerard Pde Leon,
Gilbert T Salud,
Gloria J Baca, MS,
Graham N Giesen,
Greg K Keshishyan,
Gregson A Joseph,
Gwyn G Dickinson,
Hala L Selby,
Harmon M Blanch,
Haroon Vohra (NMI),
Heika R Bounds,
Heika R Tait,
Helen B Ricalde,
Helen Verdel,
Hugh A Grimoldby,
Hugh M Mcclure, II,
Hughes,
Ian J Thomson,
Iram R Hassan, PhD,
Isaiah D Isakson,
Ivis L Negron,
Jack L Christy,
Jacob N Hamilton,
Jacqueline Mdiaz Albertini,
Jai P Singh,
James A Lane,
James C Henry,
James C Maclaughlin,
James D Hildreth,
James E Trabert,
James M Mason,
James S Cartwright,
Jane M Kreis,
Jane S Wernberg,
Janelle K Martin,
Janete F Guardia,
Jean C Colon Lopez,
Jeanne M Weishaar,
Jeff A Gard,
Jeffrey A Sommers,
Jeffrey J Leclair,
Jeffrey N Gerdes,
Jennifer C Adams,
Jennifer L Bridgewater,
Jennifer W Cheng Dobson,
Jessica B Clark,
Jinkee Mvila Binayug,
Joan Johnson,
Joan T Briones,
Joel D Martinez,
Joey V Quitania,
John Burr,
John W Banks,
Jonathan T Little,
Jorge L Guadalupe,
Jose A Lopez,
José E Meléndez,
Jose M Cayuela,
Jose O Caraballo Rivera,
Jose R Hernandez,
Joseph A Seitz, III,
Joseph M Hudak,
Julian C Hanson,
Julianne C Mccullough,
Julie A Stocklin,
Junho Pak,
Justin A Boyd,
Kari M Johansen,
Katey L Gibson,
Katharine Chan,
Katherine L Arnold,
Katherine Szestypalow,
Kathryn A Krentz,
Kathryn E King,
Keegan B Mixon,
Kelsey A Volkman,
Kelvin X Sanders,
Kenneth A Glatt,
Kenneth O Gee, PhD,
Kerry Ann Latham,
Kevin A Gonzalez,
Kevin T Gerrity,
Kham Phommachanh,
Kimberly Jproctor Jones,
Kristen L Demaio,
Kristen L Kamas,
Kristen Nickens, PhD,
Kristin M Abaonza,
Kristina M Phelps,
Kristy A Zielny,
Kristy E Zuroski,
Kun C Liu,
Lakshmi Narasimhan, PhD,
Lance A Finnical,
Lance W Bohm,
Laura Fontan, MS,
Laura Kathryn,
Laurel A Beer,
Lauren E Swantko,
Laurie Nelson,
Laurimer Kuilan Torres,
Lawrence Y Lee, PhD,
LCDR Debra Emerson,
Lenita R Silvera,
Leon R Marquart,
Lesley A Swanson,
Libia M Lugo,
Liming Zhang,
Linda F Murphy,
Linda S Leja,
Lindsey Brown, PhD,
Lisa A Bowden,
Lisa K Capron,
Lloyd D Payne,
Lori J Silverstein,
Luis A Dasta,
Luis Mburgos Medero,
Lynda L Perry, PhD,
Malinda C Shelman,
Mamta Gautam Basak, PhD,
Marcellinus D Dordunoo,
Margo C Jones,
Maria Gutierrez Lugo, PhD,
Maria Pkelly Doggett, MBA,
Marian E Ramirez,
Marie F Morin,
Marijo B Kambere, PhD,
Marion D Wimberly, Jr,
Mariza M Jafary,
Mark E Imsland,
Mark E Moen,
Mark L Collins,
Mark S Crippen,
Mark W Babbitt,
Matthew B Casale,
Matthew C Watson,
Matthew R Barr,
Maxine H Wong,
Meisha R Sampson,
Meisha Waters,
Melissa J Garcia,
Merry Christie, PhD,
Michael A Charles,
Michael E Maselli,
Michael H Antee,
Michael J Donovan,
Michael L Chasey,
Michael R Goga,
Michael S Araneta,
Michael S Hudson,
Michael Shanks, MS,
Michele Gottshall,
Mihaly S Ligmond,
Mikel T Wright,
Mikhail V Ovanesov, PhD,
Mindy M Chou,
Monica E Caphart,
Mra B Kamberem,
Mra Davism,
Mra Mcculloughj,
Muralidhara B Gavini, PhD,
Nadeem I Chaudhry,
Nancy A Saxenian Emmons,
Nancy E Byerly,
Nancy E Doyle,
Narahi J Alvarez Alcazar,
Nataniel Phillips Sylvain, PhD,
Neil J Traaen,
Nicholas L Hunt,
Nicole E Knowlton,
Norman Wong,
Omotunde O Osunsanmi,
Paraluman S Leonin,
Parul M Patel,
Patricia D Leinbach,
Paul F Rader,
Paul M Kawamoto,
Paul Rader,
Paula A Trost,
Peter E Baker,
Peter Kessler, PhD,
Peter T Regan,
Philip E Ake,
Prabhu P Raju,
Pratik S Upadhyay, DDC,
Rabia Ballica, PhD,
Rafael Nevarez Nieves,
Rajiv R Srivastava,
Ralph H Vocque,
Randy L Self,
Raquel Gonzalez Rivera,
Rebecca A Sletner,
Rebecca Rodriguez,
Rebecca T Davis,
Regina T Brown,
Reyes Candau Chacon, PhD,
Richard A Martin,
Richard G Runyon,
Richard L Friedman,
Richard Ledwidge (nmi), PhD,
Richmond K Yip,
Riley C Myers, PhD,
Rita K Kabaso,
Robert D Tollefsen,
Robert J Doyle,
Rocco C Black,
Roger F Zabinski,
Ronald E Gill,
Rowena S Nguyen,
Saied A Asbagh,
Saleem A Akhtar,
Samantha J Bradley,
Samina S Khan,
Sandra A Boyd,
Sandra A Hughes,
Sandra K Wangen,
Sangeeta M Khurana, PhD,
Sangeeta M Rataul,
Sara H Gabel,
Sarah E Mcmullen,
Sarah Kennett, PhD,
Sarah M Parkinson,
Satheesh Thomas,
Scott A Nabe,
Scott B Laufenberg,
Scott W Fox,
Shahriar Rahmani,
Shannon J Hall,
Sharon I Gundersen,
Sharon K Ferguson,
Sharon K Thoma, PharmD,
Shelagh D Schopen,
Sherri N Rohlf, MD,
Sonia R Peterson,
Sony Mathews,
Sripal R Mada, PhD,
Stacey S Degarmo,
Stacie A Woods,
Stacy A Schneider,
Stanley B Eugene, BS, BME,
Stephanie A Jameson,
Stephanie A Slater, MS,
Stephanie J Shaw,
Stephen D Brown,
Stephen J Mottola,
Stephen R Souza,
Steven C Madzo,
Steven D Kehoe,
Steven M Weinman,
Steven P Donald,
Sulene X Han,
Sundy V Sedwick,
Sunitha K Rajaram, PhD,
Susan F Laska, MS,
Susan M Jackson,
Susan P Bruederle,
Susanne M Richardson, MS RAC,
Tara M Pianko,
Ted L Anderson,
Teegan Dellibovi Ragheb, PhD,
Terri L Dodds,
Thomas C Mclean, CFS,
Thomas J Arista,
Thuy T Nguyen, LCDR,
Tina S Roecklein,
Tonia F Bernard,
Tonya R Johnson,
Tracy K Li,
Truong Xuan Nguyen (Andy),
Uttaniti Limchumroon (Tom),
V Teres Speer,
Valerie L Whipp,
Vanessa Y Gelsey, PhD,
Vanessa Y Jacobs,
Veronica L Bush,
Victor A Meo,
Virgilio F Pacio, CSO,
Vitaliy N Guyvan,
Vlada Matusovsky,
Walden H Lee,
Wayne E Seifert,
Wenzheng Wendy Zhang,
Wilfred J Omoloh,
William C Hughes,
William J Leonard,
Willis L Hicks,
Xiaojun Yan,
Xikui Chen (nmi), PhD,
Xiomara Copeland,
Yideng Liang,
Yuan Chia Kuo,
Yumi J Hiramine,
Yvesna C Blaise,
Yvonne M Larsen,
Zhong Li, PhD,
Zhongren Wu
S Lori Brown, PhD MPH's Documents
Experience Redica Systems’ NEW investigator profiles and dashboards
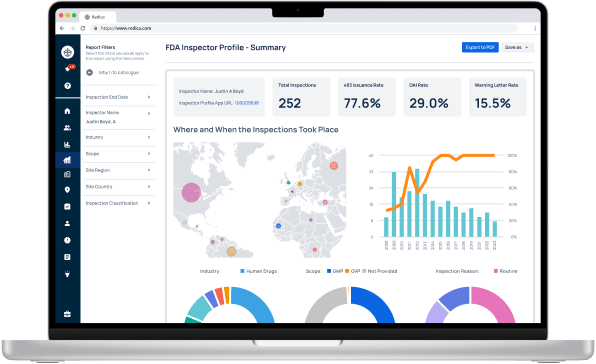
Redica Systems customers get access to over 1,400 FDA Investigator profiles, containing powerful analysis like:
- Total inspections conducted
- 483 rate
- OAI rate
- Warning Letter rate
- Inspections by Industry, Scope, and Inspection Reason
- And much more
Investigator Profiles are not sold as on-demand documents. If you’d like to see one, fill out the form below to connect with our Sales Team and get it in your inbox as little as 24 hours.
Talk to sales to access our investigator profiles
Loading...
Investigator Profiles Are Just the Tip of the Iceberg
Becoming a Redica Systems customer not only provides you with unlimited access to profiles similar to the one above for every current and former FDA Investigator, but also grants you access to:
Inspection Intelligence
- Pre-Approval Inspection (PAI) trends
- The ability to filter nearly any FDA enforcement action by GxP labels like GMP, GCP, etc.
- Latest 483s: see what the trends are across all FDA Investigators
- CFR Heatmap: Citations aggregated by Subpart by Year
Vendor Intelligence
- Full inspection histories for your vendors down to the specific site level
- All of the functionality mentioned above but isolated to just your vendors
Regulatory Intelligence
- Zero in on the trends for a specific agency/regulator (ex. FDA, MHRA, EMA, Health Canada)
- All the latest "Signals" from agencies around the world, segmented by Country, Type (ex. Guidance, Decree...), Category (ex. Rules/Regulations/Guidance, News, Reports...), Theme (ex. Cell and Gene Therapy, AI...), and more