FDA Investigator Andrea A Branche
Andrea A Branche has conducted inspections on 418 sites in 34 countries as of 20 May 2024. See below for a list of the FDA enforcement documents resulting from those inspections.
Investigator Details
Number of Inspected Sites:
418
Last Inspection Date:
20 May 2024
Investigator Role:
FDA Investigator
Redica ID:
Countries of Inspections:
United States of America,
Andorra,
Bulgaria,
Chile,
Australia,
France,
United Kingdom of Great Britain and Northern Ireland,
Italy,
India,
Germany,
Croatia,
Hungary,
Netherlands,
Belgium,
Japan,
Korea (Republic of),
China,
South Africa,
Tunisia,
Switzerland,
Denmark,
Poland,
Mexico,
Israel,
Morocco,
Malaysia,
Ireland,
Norway,
Sweden,
Singapore,
Brazil,
Philippines,
Colombia,
Zimbabwe
FDA Investigators that have inspected at least one site in common with Andrea A Branche:
Ademola O Daramola,
Akbar J Zaidi,
Akbar Javed,
Alanna L Mussawwir Bias,
Albert F Peacock, PhD,
Albinus M D'sa,
Alexander M Kay,
Allen F Hall,
Althea A Williams,
Amanda J White,
Ana M Ontiveroz,
Anastasia I Onuorah,
Andrea George, PhD,
Andrew B Bevill,
Andrew R Wasko,
Angel K Sandhu,
Angela Shepas,
Anh M Lac,
Anita Narula, PhD,
Ann L Demarco,
Annabelle Crusan, D,VM,
Anthony R Petriella,
Anya Dlockett Evans,
Aqualia L Nelson,
Araceli Rey, RN,
Arie C Menachem,
Arsen Karapetyan,
Arthur G Hurst,
Ashley A Mutawakkil,
Audrey J Yarbrough,
Azza Talaat,
Barbara A Rusin,
Barbara J Wilcox, PhD,
Barbara Janine Breithaupt,
Benjamin J Dastoli,
Bichsa T Tran,
Bonnie L Menzel,
Brenda W Uratani, PhD,
Brian J Ryan,
Brian M Campbell,
Byron Ho, DVM,
Byungja E Marciante,
Calvin B Koerner,
Camerson E Moore,
Camille D Brown,
Carl Lee,
Carla J Lundi,
Carlos Chavez,
Carol A Declouette Wilburn,
Carol Rivera Lopez, PhD,
Carole L Jones,
Cassandra L Abellard,
CDR Sean T Creighton,
Charles A Snipes, PhD,
Charles D Brown,
Charles L Larson,
Charles M Edwards,
Chelsea W Lamm,
Christina D Mello,
Christina E Muhn,
Christine E Kelley,
Christine I Shaw,
Christopher D Rush,
Christopher J Smith,
Christopher N Tran,
Christopher R Czajka,
Claudia Mperez Kasmarski,
Comyar Shoghi,
Constance M Dobbins,
Corey K Reno,
Corrine M Carter,
Courtney N Long,
Craig A Garmendia,
Craig T Rybus,
Cynthia A Harris, MD, RN,
Cynthia F Kleppinger, MD,
Damaris Y Hernandez,
Daniel J Lahar,
Daniel J Roberts,
Daniel L Aisen,
Daniel S Fam,
Danielle Lyke,
Danny D Horner,
Darla J Christopher,
Darren S Brown,
David G Whitman,
David M Beltran,
David R Heiar,
Dawn C Olenjack,
Dawn M Braswell,
Debbie J Cunningham,
Debra Lboyd Seale,
Deniza Karacic,
Dennis Cantellops Paite,
Diane L Raccasi,
Dianiris C Ayala,
Dina A Tallman,
Djamila Harouaka,
Dolores Harper,
Donna Ltartaglino Besone,
Douglas W Gronski,
Dr. Hansong Chen, PhD,
Dr. Jason R Caballero,
Dr. Mark J Seaton, PhD,
Dr. Ralph M Bernstein, PhD,
Dr. Sriram Subramaniam, PhD,
Dr. Zhihao Qiu (Peter), PhD,
Dr. Zhou Chen (nmi), MD PhD,
Dustin R Abaonza,
Dylan D Yao, MD, PhD,
Ed Gonzalez Vazquez,
Edward D Mcdonald,
Edward J Janik,
Edwin Ramos,
Elisa M Fleming,
Elizabeth D Connell,
Ellen J Kleintop,
Ellen J Tave,
Emilio O Escobar,
Emily Shacter,
Eric L Dong, BS,
Eric M Padgett,
Eric R Heebner,
Eric S Pittman,
Erin La Rue,
Ernest Serna, III,
Falana E Jackson,
Felix J Marrero,
Francisco J Mendoza,
Frank Verni,
Frans E Mercado,
Frederic W French, III,
Gajendiran Mahadevan, PhD,
Gam S Zamil,
Gene D Arcy,
Gene R Gunn,
Gerald Feldman,
Gerald M Feldman, PhD,
Gerald Mierle,
Gerald N Mcgirl, DDS,
Grace E Mcnally,
Grant L Davis,
Habacuc V Barrera,
Hala L Selby,
Hamed Ghoda,
Hasan A Irier, PhD,
Henry K Lau,
Himanshu Gupta, PhD,
Hugh M Mcclure, II,
Hung V Le,
Iris C Macinnes,
J Elkins,
Jacqueline A O'shaughnessy, PhD,
James C Maclaughlin,
James D Planchon,
James H Robinson,
James L Dunnie, Jr,
James M Odonnell,
James R Evans,
James R Fleckenstein,
Janet B Abt,
Janete F Guardia,
Janice M Hickok,
Jawaid Hamid,
Jeanne Fringer, PhD,
Jeffrey M Watson,
Jeffrey R Wooley,
Jennifer D Young,
Jennifer Hua,
Jennifer M Gogley,
Jennifer O Dowdy,
Jesse A Vazquez,
Jessica L Pressley,
Joan T Briones,
Joanne M Schlossin,
Jocelyn C Turner,
Jodi M Gatica,
Joel D Martinez,
Joey V Quitania,
Johann M Fitch,
John A Gonzalez,
John A Iwen,
John A Kadavil, PhD,
Jonathan R Campos,
Jose A Lopez,
José E Meléndez,
Jose Martinez, Jr,
Joseph E Duran,
Joseph Kutze, PhD,
Joseph P Iser, MD,
Joseph R Haynes,
Joseph R Strelnik,
Joshua S Hunt,
Jude C Dikes,
Julian C Hanson,
June P Page,
Junho Pak,
Justin A Boyd,
Justine Tomasso,
Ka L Wong,
Kaitlyn T Dang,
Kara A Scheibner, PhD,
Karen C Daugherty,
Karen K Moksnes,
Karen Takahashi,
Kari M Johansen,
Kari M Laynor,
Katherine Szestypalow,
Kathryn A Krentz,
Kellie L Thommes, RN,
Kelly D Sheppard,
Kelvin Cheung,
Kenitra D Hewitt,
Kenneth H Williams,
Kenneth R Merritt,
Kevin D Kallander,
Kevin P Foley,
Kham Phommachanh,
Khunnathee Stoner,
Kim M Downing,
Kimberly Lewandowski Walker,
Kimberly M Lichter,
Kinh Q Mac,
Kirtida Patel,
Krystal O Ogunremi,
Kwong P Lee,
L Grant,
Lakecha N Lewis,
Lakisha M Williams,
Lan T Tran,
Lance D Johnson,
Lance Mde Souza, MBA,
Lance Sindo, CIH, CQA, RS,
Latorie S Jones,
Laura Fontan, MS,
Laureen M Geniusz,
Lauren E Skokan,
Laurie P Norwood,
LCDR Angelica M Chica,
LCDR Jennifer H Rhyu,
Leighton Kn Gai,
Leo J Lagrotte,
Leonard H Lavi,
Li Hongpaul Yeh, PhD,
Liliane Brown,
Lillie D Witcher,
Lillie M Young,
Linda R Kuchenthal,
Linda Thai,
Linh Tu,
Lisa A Doyle,
Lisa M Lopez,
Lisa M Puttonen,
Lisa R Hilliar,
Lisa R Jennings,
Lisa R Whitt,
Lisa Shin,
Lloyd D Payne,
Lori A Gioia,
Lori M Graham,
Lorie S Hannappel,
Lucas B Leake,
Luis Mburgos Medero,
Lynda L Perry, PhD,
Mabel M Lee,
Maida Henesian (NMI),
Maira P Brading,
Makini Cobourne Duval, PhD,
Marc R Dickens,
Marcellinus D Dordunoo,
Marcus F Yambot,
Margaret M Annes,
Marie B Buen Bigornia,
Marie K Kinkade,
Mariza M Jafary,
Mark C Flotte,
Mark W Babbitt,
Marsha W Major,
Martin K Yau, PhD,
Martina E Lagrange,
Maryam Tabatabaie,
Massoud Motamed,
Matthew J Sienko,
Matthew M Vernon,
Maya M Davis,
Melanie W Pishnery,
Michael A Charles,
Michael E Maselli,
Michael F Skelly, PhD,
Michael J Nerz,
Michael Shanks, MS,
Michael T Cyrus,
Michelle J Glembin,
Michelle J Hines,
Michelle M Noe Varga,
Michelle Rfrazier Jessen, PhD,
Mihaly S Ligmond,
Min Shanmabel Liu,
Mohsen Rajabi Abhari, FDA,
Monica J Wilkins,
Monique Rac Lester,
Mra B Kamberem,
Mra Davism,
Nancy A Bellamy,
Natalie A Mickelsen, DVM,
Natalie J Ayoub,
Navista C Bolton,
Nayan J Patel,
Nicholas L Hunt,
Nicholas Obiri, PhD,
Nicola M Fenty Stewart,
Nicole E Knowlton,
Niraj R Mehta, PhD,
Oluwasefunm Agbanigo,
Omotunde O Osunsanmi,
Paquita F Segarra,
Patrice S Hall,
Patricia M Jacobs,
Patrick D Stone, MS,
Patrick J Mcneilly,
Patty P Kaewussdangkul,
Paul A Bonneau,
Paul E Stein,
Paul P Geraci,
Paula J Perry,
Pauline N Logan,
Peter E Baker,
Peter Kessler, PhD,
Philip M Steele, PhD,
Phillip D Waldron,
Phillip M Pontikos,
Pitman,
Rachel M Skeete,
Rafael H Ortiz,
Ralph H Vocque,
Ramon Lgonzalez Prieto,
Raymond T Oji,
Reba A Gates,
Rebecca Co Bryan,
Rebecca L Stephany,
Rebecca Rodriguez,
Rebecca T Davis,
Regina T Brown,
Reyes Candau Chacon, PhD,
Rhonda L Dash,
Richard Jay Tucker, RS,
Richard K Vogel,
Richard L Rutherford,
Riley C Myers, PhD,
Robert Barron,
Robert D Tollefsen,
Robert M Tillman,
Robert T Lorenz,
Rodney L Tinzie,
Ron Mabrysmelz,
Ronald C Mabry Smith,
Ronald T Weber,
Ronda Rloyd Jones,
Rose Ashley,
Roy Baby,
Roy R Rinc,
Ruth E Medcalf,
S Lori Brown, PhD MPH,
Sam H Haidar, PhD,
Samina S Khan,
Samson O Oluseye,
Sandra S Saniga,
Sandy J Ziegler,
Sangeeta M Khurana, PhD,
Scott B Laufenberg,
Scott K Zika,
Scott N Lim,
Scott R Nichols, PhD,
Scott T Ballard,
Seema S Singh,
Selene T Torres,
Serge L Beaucage,
Sha'tina R Alridge,
Shaquenta Y Perkins,
Sharon M Harold,
Sharyn K Miller,
Shawn E Larson,
Sheilyn H Huang,
Shelby N Turner,
Shelley H Beausoleil,
Sherri J Jackson,
Sidney B Priesmeyer,
Sonia R Peterson,
Sony Mathews,
Sripal R Mada, PhD,
Staci G Mcallister,
Stacie A Woods,
Stephanie A Slater, MS,
Stephen D Brown,
Stephen D Eich,
Stephen L Beekman,
Stephen R Souza,
Steven Fong, MS, PhD,
Stuart W Russell,
Susanne M Richardson, MS RAC,
Suzanne N Vallez,
Tawny L Colling,
Teresa I Navas,
Terri E Gibson,
Terri L Dodds,
Terry C Kimball,
Thai T Duong,
Thao T Kwan,
Thao X Tran,
Thea C Grome,
Thomas J Arista,
Thuy T Nguyen, LCDR,
Timothy C Grome,
Tina M Pawlowski,
Tonia L Sawyer,
Torrance J Slayton,
Tracey Asinjen Wiersma,
Travis M Beard,
Tricia S Martinez,
Tricia Y Samaniego,
Trudy R Papson,
Truong Xuan Nguyen (Andy),
Veronica L Bush,
Victor Spanioli,
Virgilio F Pacio, CSO,
Vivian Garcia,
Walden H Lee,
Wayne E Seifert,
Wen Ning Chan (Sally),
William D Tingley,
William J Leonard,
William V Millar,
Xiaojun Yan,
Xiaoping Guan,
Xikui Chen (nmi), PhD,
Yi Wang, PhD,
Young M Choi, PhD,
Young M Yoon,
Yvette E Guillermo,
Yvette S Montes,
Yvonne T Lacour,
Zerita White
Andrea A Branche's Documents
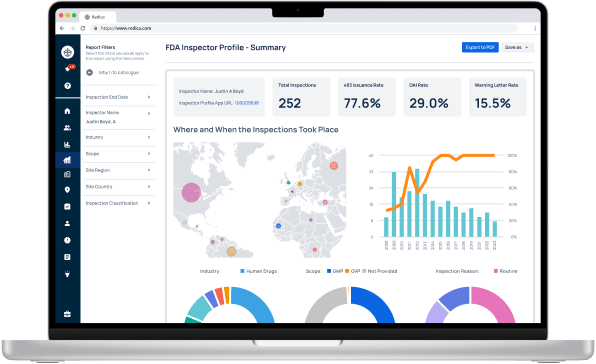
Experience Redica Systems’ NEW investigator profiles and dashboards
Redica Systems customers get access to over 1,400 FDA Investigator profiles, containing powerful analysis like:
- Total inspections conducted
- 483 rate
- OAI rate
- Warning Letter rate
- Inspections by Industry, Scope, and Inspection Reason
- And much more
Investigator Profiles are not sold as on-demand documents. If you’d like to see one, fill out the form below to connect with our Sales Team and get it in your inbox as little as 24 hours.
Talk to sales to access our investigator profiles
Loading...
Investigator Profiles Are Just the Tip of the Iceberg
Becoming a Redica Systems customer not only provides you with unlimited access to profiles similar to the one above for every current and former FDA Investigator, but also grants you access to:
Inspection Intelligence
- Pre-Approval Inspection (PAI) trends
- The ability to filter nearly any FDA enforcement action by GxP labels like GMP, GCP, etc.
- Latest 483s: see what the trends are across all FDA Investigators
- CFR Heatmap: Citations aggregated by Subpart by Year
Vendor Intelligence
- Full inspection histories for your vendors down to the specific site level
- All of the functionality mentioned above but isolated to just your vendors
Regulatory Intelligence
- Zero in on the trends for a specific agency/regulator (ex. FDA, MHRA, EMA, Health Canada)
- All the latest "Signals" from agencies around the world, segmented by Country, Type (ex. Guidance, Decree...), Category (ex. Rules/Regulations/Guidance, News, Reports...), Theme (ex. Cell and Gene Therapy, AI...), and more