FDA Investigator Dr. Mark J Seaton, PhD
Dr. Mark J Seaton, PhD has conducted inspections on 94 sites in 6 countries as of 06 May 2024. See below for a list of the FDA enforcement documents resulting from those inspections.
Investigator Details
Number of Inspected Sites:
94
Last Inspection Date:
06 May 2024
Investigator Role:
FDA Investigator
Redica ID:
Countries of Inspections:
United States of America,
India,
China,
Austria,
Canada,
Taiwan
FDA Investigators that have inspected at least one site in common with Dr. Mark J Seaton, PhD:
Abdellah El Hajjam,
Alan D Centi,
Alexandra B Pitkin,
Alicia M Mozzachio,
Amber G Chung,
Amber G Wardwell,
Amir Alavi,
Ana P Barido,
Anastasia I Offordile,
Andrea A Branche,
Andrea George, PhD,
Andrew M Barlow,
Andrew R Wasko,
Andria L Kuhlman,
Angel K Sandhu,
Angela Shepas,
Anh M Lac,
Anita R Michael,
Ann Marie Karnick,
Ann Marie Montemurro,
Anna M Brannen,
Anthony A Charity,
Arianne L Motter,
Arindam Dasgupta, PhD,
Atul J Agrawal,
Austin B Appler,
Barbara A Rusin,
Barbara J Holladay,
Barbara J Rincon,
Barbara J Wilcox, PhD,
Barbara Janine Breithaupt,
Betsy C Galliher,
Bichsa T Tran,
Binh T Nguyen,
Brandi L Garbutt,
Brandon J Brookens,
Brandon L Mariner,
Brandy N Lepage,
Brian D Young,
Byron Ho, DVM,
Byungja E Marciante,
Calvin B Koerner,
Calvin W Edwards,
Cara M Minelli,
Carl A Anderson,
Carl Lee,
Carla C Tuite,
Carmen Y Fisher,
Carol Rivera Lopez, PhD,
Carole L Jones,
Carrie A Hughes,
Caryn M Everly,
Caryn M Mcnab,
CDR Michael J Mero,
Chaltu Nwakijra,
Chamberlain,
Charles A Snipes, PhD,
Charles R Bonapace, PhD,
Charles R Cote, RIC,
Charlotte E Wilkins,
Chase Bourke, PhD,
Chelsea W Lamm,
Ching Jeyg Chang,
Christian D Lynch (CDL),
Christina A Miller,
Christina K Theodorou,
Christine M Whitby, CSO,
Christopher R Czajka,
Christopher T Middendorf,
Claudia Wrzesinski,
Clinton D Priestley,
Connie P Rezendes,
Corey K Reno,
Courtney N Long,
Craig T Rybus,
Crayton Lee,
Crystal A Harlan,
Ct Viswanathan, PhD,
Cynthia A Harris, MD, RN,
Cynthia Jim, CSO,
Cynthia L Rakestraw,
Cynthia White,
Daniel J Roberts,
Daniel R Azar,
Daniel R Tammariello,
Daniel W Cline,
Darin S Wiegers,
Dauksism,
David J Hafner,
David K Glasgow,
David L Chon,
David L Pearce,
David P Vanhouten,
Dawn C Olenjack,
Dawn D Aiello,
Deborah A Greco,
Deborah G Haney Cozzone,
Deborah H Cozzone,
Debra Bower,
Debra J Bennett,
Debra Lboyd Seale,
Dell S Moller,
Denise L Burosh,
Diane Cvan Leeuwen,
Dina A Tallman,
Donald B Mckechnie,
Douglas A Campbell,
Douglas W Gronski,
Dr. Abhijit Raha, PhD,
Dr. Gopa Biswas, PhD,
Dr. Hansong Chen, PhD,
Dr. Ralph M Bernstein, PhD,
Dr. Sriram Subramaniam, PhD,
Dr. Zhou Chen (nmi), MD PhD,
Dustin P Tran,
Dustin R Abaonza,
Dylan D Yao, MD, PhD,
Edward D Mcdonald,
Eias A Zahalka,
Eileen A Liu,
Emest F Bizjak,
Emily Shacter,
Eric L Dong, BS,
Eric S Myskowski,
Eric S Pittman,
Erica L Nicoll,
Erik A Bungo,
Erika M Wilkerson,
Erin L Mcfiren,
Erin M Mcdowell,
Farhana Khan,
Francis J Eng,
Frederick M Lochner,
Gabrielle J Swain,
Gajendiran Mahadevan, PhD,
Gary J Hagan,
Gayle S Lawson,
Geoffrey K Kilili,
George Pyramides,
Gerald Feldman,
Gerald M Feldman, PhD,
Gerald W Kopp,
Gloria A Milster,
Grace P Santos,
Greg K Keshishyan,
Gregory E Beichner,
Gregory W Smith,
Gwyn G Dickinson,
Hanna L Potter,
Heika R Bounds,
Heika R Tait,
Henry K Lau,
Himanshu Gupta, PhD,
Hugh M Mcclure, II,
Hyojong Kwon, PhD,
Jacqueline A O'shaughnessy, PhD,
Jacqueline Mdiaz Albertini,
James M Mason,
James P Mcevoy,
James P Stumpff,
James R Evans,
James W Plucinski,
Jane M Kreis,
Janet L Bowen,
Janete F Guardia,
Jasjeet K Sekhon,
Jeanne Fringer, PhD,
Jeanne J Thai,
Jeffrey M Watson,
Jennifer C Adams,
Jennifer C Johnson,
Jennifer L Jager,
Jennifer M Gogley,
Joan A Loreng,
Joan T Briones,
Joanne Hajoway,
Joanne M Hajoway,
Joanne M Schlossin,
Jocelyn E Massey,
Jocelyn E Sparks,
Joey V Quitania,
Jogy George,
Johann M Fitch,
John A Kadavil, PhD,
Jonathan R Campos,
Jose M Cayuela,
Joseph Kutze, PhD,
Joseph L Despins, PhD,
Joseph P Iser, MD,
Joseph R Haynes,
Joseph T Dougherty,
Joshua S Hunt,
Juanita Banuelos,
Judith A Paterson,
Julian C Hanson,
Julianne C Mccullough,
Julie D Bringger,
Junho Pak,
Jyoti B Patel, PhD,
Ka L Wong,
Kara A Scheibner, PhD,
Karen M Kondas,
Katherine E Jacobitz,
Kathleen S Tormey,
Kathryn A Krentz,
Kellia N Hicks,
Kelly N Kerr,
Kendra L Brooks,
Kenneth Nieves,
Kent A Conforti,
Kent C Faul,
Kerun Hardeo,
Kevin P Foley,
Kim Lthomas Cruse,
Kimberly A Dux,
Kristen D Evans,
Kristina J Donohue,
Kristina Mjoyce Pittman,
Kujtim Sadiku,
Kurt A Brorson, PhD,
Laine P Myers,
Lakisha M Williams,
Lance Mde Souza, MBA,
Laurie P Norwood,
LCDR Jennifer H Rhyu,
LCDR Margaret Edi Gennaro,
LCDR Susan E Polifko,
LCDR William H Bender,
Li Hongpaul Yeh, PhD,
Linda S Leja,
Linda Thai,
Linh Tu,
Lisa Hayka,
Lori J Silverstein,
LT John M Mastalski,
Luann Mckinney,
Lynda L Lanning,
Lynette P Salisbury,
Mabel M Lee,
Maida Henesian (NMI),
Makini Cobourne Duval, PhD,
Marcelo O Mangalindan, Jr,
Marcia A Worley,
Maria A Reed,
Maria Pkelly Doggett, MBA,
Marie K Kinkade,
Marijo B Kambere, PhD,
Mariza M Jafary,
Marsha W Major,
Martha Sullivan Myrick,
Martin J Guardia,
Martin K Yau, PhD,
Mary E Storch,
Mary Jeanet Mcgarry,
Matthew C Watson,
Maureen A Duckworth,
Maureen A Wentzel,
Melissa M Schafer,
Meredith L Sheridan,
Michael A Taylor,
Michael F Skelly, PhD,
Michael J Diskin,
Michael J Nerz,
Michael Serrano,
Michael Shanks, MS,
Michael T Cyrus,
Michele M Falchek,
Michelle J Hines,
Michelle Rfrazier Jessen, PhD,
Mihaly S Ligmond,
Mike M Rashti,
Min Shanmabel Liu,
Minh D Phan,
Morris Paul,
Muralidhara B Gavini, PhD,
Myrick,
Nancy A Bellamy,
Nancy L Neiger,
Natalie A Mickelsen, DVM,
Nathan R Moon,
Nayan J Patel,
Nebil A Oumer,
Nicholas L Hunt,
Nicholas Obiri, PhD,
Nicole C Victoria, PhD,
Niraj R Mehta, PhD,
Norman K Starks,
Nutan Mytle,
Omotunde O Osunsanmi,
Paquita F Segarra,
Peter E Baker,
Peter Kessler, PhD,
Philip J Snoy,
Phillip M Pontikos,
Prabhu P Raju,
Rachel C Harrington,
Rafael Padilla,
Raymond T Oji,
Rebecca Rodriguez,
Rebecca T Davis,
Richard Heath Coats,
Richard W Berning,
Riley C Myers, PhD,
Robert B Shibuya, MD,
Robert D Tollefsen,
Robert L Hummel,
Roberta W Cunningham,
Ronald R Ruff,
Rory K Geyer,
Rosanna M Goodrich,
Rose Ashley,
Rose Ljean Mary,
Ruth A Williams,
Sabine Francke,
Sandra A Hughes,
Sandra S Saniga,
Scott B Laufenberg,
Scott N Lim,
Sean D Duke,
Seng Ching Hwang,
Seongeun Cho (Julia), PhD,
Serge L Beaucage,
Sheilyn H Huang,
Shelley H Beausoleil,
Sherri J Jackson,
Sherry L Secrist, BLT DO,
Sidney B Priesmeyer,
Sony Mathews,
Sripal R Mada, PhD,
Stacey A Priest,
Stanley Au,
Stephanie A Slater, MS,
Stephanie L Shapley,
Stephanie Mangigian, MS/OSH, RN,
Stephen J Kilker,
Steven P Donald,
Stuart W Russell,
Subramanian Muthukkumar, PhD,
Sunitha K Rajaram, PhD,
Susan D Yuscius,
Susan F Laska, MS,
Susan M Jackson,
Susan T Hadman,
Susanne M Richardson, MS RAC,
Tahseen Mirza,
Tammy L Chavis,
Teena H Aiken,
Thea C Grome,
Theresa Kirkham (NMI),
Theressa B Smith,
Thomas E Friel,
Thomas W Nojek,
Thunder N Dunkijacobs,
Thuy T Nguyen, LCDR,
Tiffani D Wilson,
Timothy C Grome,
Todd J Maushart,
Tong Zhou,
Toyin B Oladimeji,
Tracey Asinjen Wiersma,
Truong Xuan Nguyen (Andy),
Uttaniti Limchumroon (Tom),
Van Cliff,
Vashti E Bocker,
Vickie J Kanion,
Vickie L Anderson,
Victor J Gangi,
Vlada Matusovsky,
Walden H Lee,
Whitney S Reynolds,
William C Brenneman,
William D Tingley,
William J Leonard,
William V Millar,
Xiaohan Cai, PhD,
Xikui Chen (nmi), PhD,
Yangmin Ning,
Yi Wang, PhD,
Yiyue Zhang (nmi), PhD,
Young M Yoon,
Yvette I Henry,
Yvette I Johnson,
Yvette Mlacour Davis,
Zhongren Wu
Dr. Mark J Seaton, PhD's Documents
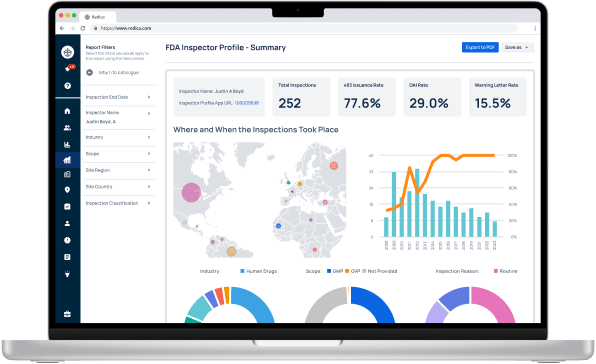
Experience Redica Systems’ NEW investigator profiles and dashboards
Redica Systems customers get access to over 1,400 FDA Investigator profiles, containing powerful analysis like:
- Total inspections conducted
- 483 rate
- OAI rate
- Warning Letter rate
- Inspections by Industry, Scope, and Inspection Reason
- And much more
Investigator Profiles are not sold as on-demand documents. If you’d like to see one, fill out the form below to connect with our Sales Team and get it in your inbox as little as 24 hours.
Talk to sales to access our investigator profiles
Loading...
Investigator Profiles Are Just the Tip of the Iceberg
Becoming a Redica Systems customer not only provides you with unlimited access to profiles similar to the one above for every current and former FDA Investigator, but also grants you access to:
Inspection Intelligence
- Pre-Approval Inspection (PAI) trends
- The ability to filter nearly any FDA enforcement action by GxP labels like GMP, GCP, etc.
- Latest 483s: see what the trends are across all FDA Investigators
- CFR Heatmap: Citations aggregated by Subpart by Year
Vendor Intelligence
- Full inspection histories for your vendors down to the specific site level
- All of the functionality mentioned above but isolated to just your vendors
Regulatory Intelligence
- Zero in on the trends for a specific agency/regulator (ex. FDA, MHRA, EMA, Health Canada)
- All the latest "Signals" from agencies around the world, segmented by Country, Type (ex. Guidance, Decree...), Category (ex. Rules/Regulations/Guidance, News, Reports...), Theme (ex. Cell and Gene Therapy, AI...), and more