FDA Investigator Zachary A Bogorad
Zachary A Bogorad has conducted inspections on 235 sites in 15 countries as of 15 Feb 2024. See below for a list of the FDA enforcement documents resulting from those inspections.
Investigator Details
Number of Inspected Sites:
235
Last Inspection Date:
15 Feb 2024
Investigator Role:
FDA Investigator
Redica ID:
Countries of Inspections:
Germany,
Spain,
United States of America,
Canada,
China,
Israel,
India,
Czechia,
Romania,
United Kingdom of Great Britain and Northern Ireland,
Norway,
South Africa,
Switzerland,
Mexico,
Italy
FDA Investigators that have inspected at least one site in common with Zachary A Bogorad:
Adam R Cooke,
Ademola O Daramola,
Adree N Anderson,
Adrienne Morgan,
Alan E Mehl,
Alan L Truong,
Alan P Kurtzberg,
Albert C Cheng,
Alex M Viehmann,
Alexandra B Pitkin,
Alice S Tsao,
Alicia M Frees,
Alicia M Mozzachio,
Alysia C Alger,
Amalia C Himaya,
Amanda E Lewin, PhD,
Amanda N Schaupp,
Amir A Abdalla,
Amy E Devine,
Ana Djurdjevic,
Ana P Pineda Zavaleta,
Ana Paulap Sandee,
Anastasia M Shields,
Anderson,
Andrea S Heise,
Andrew A Hoopes,
Andrew K Schaal,
Andrew Le,
Andy B Lee,
Angela Shepas,
Anita Narula, PhD,
Anna L Tucker,
Anney Lin,
Anthony A Charity,
Anthony F Lorenzo,
Anthony J Lagreca,
Anthony R Bucks,
April P Shaw,
Aqualia L Nelson,
Aref Mel Demerdash,
Arie C Menachem,
Armando Chavez, MS,
Arsen Karapetyan,
Ashley A Mutawakkil,
Avery J Dennis,
Azza Talaat,
Babajide Michael Osunsanmi,
Barbara I Rogolsky,
Barbara J Rincon,
Barbara Janine Breithaupt,
Barbara M Frazier,
Betty Kay Baxter,
Bichsa T Tran,
Bijoy Panicker,
Binh T Nguyen,
Blondell W Johnson,
Bonnie I Needleman,
Brandi E Williams,
Brenda C Hamilton,
Brenda W Uratani, PhD,
Brent T Hall,
Brent W Higgs,
Brett R Havranek,
Brian J Ryan,
Brian Ravitch,
Brien C Fox,
Brittany D Terhar,
Bruce E Taylor,
Bruce H Mccullough,
Bryan A Galvez,
Bryan S Roddy,
Bryce A Hammer,
Burnsb,
Bush,
Candace S Tucker,
Capri R Woolridge,
Carl A Huffman, III,
Carl J Montgomery,
Carla J Lundi,
Carla R Hinz,
Carlos Chavez,
Carmen Y Fisher,
Carolina D Vasquez,
Caroline H Le,
Carrie A Hughes,
Caryn M Everly,
Caryn M Mcnab,
Catherine J Laufmann,
Cathleen A Carr Sharpe,
CDR Rochelle B Young, RPh, MSA,
Cecilia H Kieu,
Celena Ngo,
Chad W Rice,
Chani Broner, PhD,
Chaoying C Ma,
Charles I Ann,
Charles R Cote, RIC,
Chelsea N Sealey,
Cheryl A Clausen,
Chiaochun J Wang,
Chilton L Ng,
Chris A Sack,
Christian D Lynch (CDL),
Christina Capacci Daniel, PhD,
Christine R Casey,
Christopher D Leach,
Christopher D Washington,
Christopher M Jenner,
Christopher R Czajka,
Claudia Mperez Kasmarski,
Cody D Rickman,
Cody J Alley,
Collins M Mason,
Comyar Shoghi,
Concepcion Cruz, Jr,
Conner N Mann,
Conor J Mccarron,
Courtney R Bratina,
Courtney R Ingalsbe,
Crystal Monroy,
Cynthia C Smith, PhD,
Cynthia Jim, CSO,
Cynthia L Evitt,
Cynthia M Goudeau,
Dana D Carter, SR DDS,
Danial S Hutchison,
Daniel J Lahar,
Daniel R Hurt,
Daniel Velasquez,
Darrah,
Darren S Brown,
Darrin E Davis,
Darrin W Freeman,
David A Foran,
David A Oluwo,
David D Anderson, PhD,
David F Graham,
David G Whitman,
David J Eide,
David P Vanhouten,
David Perkins,
David Serrano,
Davis,
Dawn C Olenjack,
Deborah A Greco,
Deborah W Hsu,
Debra I Love,
Dennis Cantellops Paite,
Desiree C Iya,
Deyaa Shaheen,
Diana K Krepel,
Diana M Rand,
Diane Cvan Leeuwen,
Diane R Weidley,
Dipesh K Shah,
Djamila Harouaka,
Dogbeda F Mackenzie,
Don H Bark, PhD,
Donald L Lech,
Dongping Dai, PhD,
Donna Ltartaglino Besone,
Dr. Carmelo Rosa,
Dr. Chunchang Fang,
Dr. Robert C Horan, MD,
Dr. Sriram Subramaniam, PhD,
Dr. Zhihao Qiu (Peter), PhD,
Durell L Giles,
Dustin M James,
Dwight D Ferguson,
Dyana K Stone,
Edith M Gonzalez,
Edward E Lockwood (EEL),
Edwin Melendez,
Eileen T Dupont,
Elaine S Chow,
Elizabeth C Ajuzie,
Elizabeth D Connell,
Elizabeth D Gonzales,
Ellen N Stewart,
Emilie E Kahn,
Eric C Fox,
Eric M Mueller, PharmD,
Eric M Padgett,
Erickson,
Erika V Butler,
Erin C Dugan,
Esteban Beltran,
Esther B Gamallo Herrera,
Esther C Broner, PhD,
Evelyne L Weaver,
Farhana Khan,
Felix Maldonado,
Francis A Guidry,
Francisco,
Frank Wackes,
Franklin D Heisler,
Gabriel Craig,
Gam S Zamil,
Garciam,
Garrad R Poole,
Gary R Wong,
Gavin T Cua,
Gene D Arcy,
Geoffrey K Wong,
Geoffrey M Leist,
George Lunn, PhD,
Georgia A Layloff,
Gerard T Schneider,
Ginger M Sykes,
Glen C Lawrence,
Gloria J Baca, MS,
Gloria J Champagne,
Gonzalo Zendejas,
Grace E Mcnally,
Grace P Santos,
Greg K Keshishyan,
Gretchen Lf Trendel,
Gunneet Kaur,
Gwyn G Dickinson,
Haroon Vohra (NMI),
Heath W Cartwright,
Hector Jcolon Torres,
Heidy C Perales,
Helen Verdel,
Henry E Carrillo,
Herminio C Francisco,
Hugh A Grimoldby,
Huiquan Wu,
Hung H Do, MS,
Huy T Mai,
Ingrid Y Johnson,
Isabel Y Espinosa,
Ivis L Negron,
J Michael Cannon, PhD,
Jacob G Lutz,
Jacob W Reynolds,
James A Barreto,
James D Bridges,
James D Planchon,
James I Giefer,
James K Rice,
James L Dunnie, Jr,
James M Mason,
James P Mcreavey,
James P Stallings,
James P Stumpff,
Jamie L Dion,
Janet Pulver,
Janete A Eaker,
Janete A Oliveira,
Janete F Guardia,
Jazmine M Welcher,
Jeannie M Vonderbrink,
Jeannie Pradyanata,
Jeff M Uriarte,
Jeffery A Johnson,
Jeffrey B Moody,
Jeffrey D Meng,
Jeffrey P Raimondi,
Jennie J Fan,
Jennifer A Hickey,
Jennifer A Robinson,
Jennifer L Schmidt,
Jennifer M Gogley,
Jennifer M Pouget,
Jeremy Beber,
Jessica E Hensley,
Jessica L Andrade,
Jessica L Pressley,
Jill J Tillman,
Jimmie L Weigand,
Jinnie Kokiatkulkij,
Joan A Loreng,
Joan M Cantellops Figueroa,
Joan M Nandrea,
Joanne E King,
Jocelyn E Massey,
Joel D Hustedt,
Joey V Quitania,
Jogy George,
John A Daubenspeck,
John A Gonzalez,
John A Marchin,
John C Mcmichael,
John Cook,
John P Jorgensen,
John T Vonderbrink,
Johnson,
Jolanna A Norton,
Jonathan K Dare,
Jonathan R Campos,
Joohi Castelvetere,
Jorge L Bertran,
Jose A Lopez,
Jose Acruz Gonzalez,
José E Meléndez,
Jose M Cayuela,
Joseph C Yen,
Joseph R Lambert,
Joseph R Strelnik,
Joshua P Wireman,
Juandria V Williams,
Judy C Nepsa,
Julia Ventura,
Julie A Rorberg,
Julie A Stocklin,
Julie N Vosilus,
Justin A Boyd,
Justin R Carr,
Ka L Wong,
Kara L Roden,
Karen C Daugherty,
Karen G Hirshfield,
Karen J Bak,
Karen M Cruz Arenas,
Karen P Langer,
Kari M Johansen,
Karthik B Iyer,
Katherine E Jacobitz,
Katherine M Taylor,
Katherine Szestypalow,
Kathleen B Swat,
Kathleen J Close,
Kathleen M Bradley,
Kathleen S Tormey,
Kathryn M Mogen,
Keeshunna D Daniels,
Kelley,
Kellia N Hicks,
Kelly D Moore,
Kelvin X Sanders,
Kenneth D Bland,
Kenneth H Williams,
Kenneth O Gee, PhD,
Kenneth S Boehnen,
Kent C Faul,
Kevin A Gonzalez,
Kevin Lee,
Kevin T Gerrity,
Kham Phommachanh,
Khanh T Van,
Khoa Nathanv Tran,
Kim Lthomas Cruse,
Kimberlee A Ewing,
Kimberley A Hoefen,
Kimberly M Lichter,
Kirtida Patel,
Ko U Min,
Kristen E Szenderski,
Kristi B Panzer,
Kristi M Hampton Thurston,
Kristin M Abaonza,
Kumar G Janoria,
Lakecha N Lewis,
Lance A Finnical,
Lance Mde Souza, MBA,
Lane V Christensen, PhD,
Lanita F Kelley,
Larisa E Pavlick,
Lata C Mathew, PhD,
Laura B Kennedy,
Laura Fontan, MS,
Laura S Huffman,
Laurimer Kuilan Torres,
Lavender M Huskey,
LCDR Chad N Thompson,
LCDR Debra Emerson,
LCDR Ismael Olvera, IV,
LCDR Jennifer H Rhyu,
LCDR Matthew J Morrison,
Lena Nguyen,
Leonard H Lavi,
Lesley Maep Lutao,
Leticia I Romero,
Li Hongpaul Yeh, PhD,
Lillian S Wu,
Lilly O Barton,
Liming Zhang,
Linda Cherry,
Linda F Murphy,
Linda M Cheny,
Linda R Kuchenthal,
Linda Thai,
Lindsey M Schwierjohann,
Ling Yu L Liu,
Linhart,
Lionell L Thomas,
Lisa D Ellis,
Lisa K Capron,
Lisa L Flores,
Lisa L Gilliam,
Lisa Shin,
Lloyd M Luapula,
Loretta J Jordan,
Lori A Gioia,
Lori J Silverstein,
Louis B Cencetti,
Louis S Chen,
Lucas B Leake,
Lucila B Nwatu,
Luis Mburgos Medero,
Lyndsey G Smith,
Lynn L Wong,
Maan S Abduldayem,
Mai X Huynh,
Maida Henesian (NMI),
Makini Cobourne Duval, PhD,
Marc A Jackson, Jr,
Marco S Esteves,
Marcus A Ray,
Marcus F Yambot,
Margaret A Smithers,
Margaret E Walsh,
Margaret M Annes,
Margaret M Sands,
Maria Estrella,
Marie B Buen Bigornia,
Marie Elena C Puleo,
Marie F Morin,
Marijo B Kambere, PhD,
Marion D Wimberly, Jr,
Marion W Nadeau,
Mariza M Jafary,
Mark C Saale,
Mark S Ross,
Marlene G Swider,
Martin K Yau, PhD,
Mary K Concannon,
Matthew A Johnson,
Matthew B Casale,
Matthew C Watson,
Matthew H Hunt,
Matthew J Hansen,
Matthew J Johnson,
Matthew M Vernon,
Matthew Ondeck,
Matthew R Clabeaux,
Matthew R Dionne,
Matthew R Sleeter,
Maurice M Sheehan,
Maxyne T Lam,
Maya M Davis,
Megan M Kirk,
Meisha Waters,
Melanie C Voges,
Melinda L Rice,
Melisa A Healy,
Melissa D Ray,
Melissa N Simanton,
Melva J Palmer,
Metitia M Gramby,
Michael A Charles,
Michael A Feingold,
Michael A Taylor,
Michael C Zubelewicz,
Michael D Garcia,
Michael E Maselli,
Michael J Kuchta,
Michael R Goga,
Michael S Araneta,
Michael S Call,
Michael S Smoker,
Michele L Obert,
Michele Perry Williams,
Michelle J Hines,
Miguel A Martinez Perez,
Mihaly S Ligmond,
Minerva Rogers,
Minh D Phan,
Miranda D Mcdonald,
Mohsen Rajabi Abhari, FDA,
Monica Cburgos Garcia,
Monica M Mcclure,
Monique M Brooks,
Mra Davism,
Mra Mcculloughj,
Mra Munizn,
Muralidhara B Gavini, PhD,
Myers,
Nadine Nanko Johnson,
Nancy E Byerly,
Nancy E Smith,
Nancy G Schmidt,
Nancy L Rolli,
Naseem S Jouhari,
Natalie J Ayoub,
Natalie J Reese,
Nataniel Phillips Sylvain, PhD,
Nathan M Jornod,
Nathan R Moon,
Naveen B Walker,
Nayan J Patel,
Neal L Adams,
Ngoc T Le,
Nia K Walker,
Nianna C Burns,
Nicholas A Violand,
Nicholas L Hunt,
Nicholas Z Lu,
Nicole J Conklin,
Noreen Muñiz,
Omotunde O Osunsanmi,
Paige E Shelborne,
Paige E Wilson,
Pal S Mayasandra,
Parul M Patel,
Patricia A Brown,
Patricia A Cortez,
Patricia D Stahnke,
Patricia F Alberico,
Patricia L Payne,
Patrick C Klotzbuecher,
Patrick L Wisor,
Paul L Bellamy,
Paula M Laplant,
Peter E Gruman,
Peter R Lenahan,
Phal K Chhun,
Philip F Istafanos, DMV, MS,
Philip Kreiter,
Phillip J Sample,
Phillip L Toy,
Phillip M Pontikos,
Prabhu P Raju,
Quynh H Strandberg,
Rachel Collen,
Rafael Nevarez Nieves,
Ralph H Vocque,
Randall N Johnson,
Randy D Moore,
Randy L Self,
Rashonda N Rucker,
Raymond T Anderson,
Raymond T Oji,
Raymond Valdivia,
Rebecca Rodriguez,
Richard C Chiang,
Richard D Coleman,
Richard W Tubb,
Richmond K Yip,
Rick L Friedman,
Ricki A Chase,
Rita K Kabaso,
Robert C Hoover,
Robert D Tollefsen,
Robert J Doyle,
Robert J Ham,
Robert R Wilson,
Robert T Lorenz,
Robin K Hatfield,
Robin K Reel,
Rochelle A Rolnik,
Rochelle L Cross,
Rocio Guzman Velazquez,
Rodney D Combs,
Rodney G Raiford,
Roger F Zabinski,
Roger L Farmer,
Rohit B Kolhatkar,
Rose Ashley,
Rose Xu,
Rowena S Nguyen,
Roy R Rinc,
Rumany C Penn, PharmD,
Russell J Glapion,
Ryan J Borges,
Ryan S Martin,
Saied A Asbagh,
Sam H Haidar, PhD,
Samantha J Bradley,
Sandra A Boyd,
Sandra A Hughes,
Sandra S Saniga,
Sangeeta M Khurana, PhD,
Santiago Gallardo Johnson,
Santos E Camara,
Sara H Gabel,
Sara Jdent Acosta,
Sarah A Hassas,
Sarah E Morris,
Scott J Lewis,
Scott J Matsuda,
Scott N Lim,
Sean M Tessicini,
Sean P Desbrow,
Sean R Marcsisin,
Secrist,
Selene T Torres,
Shafiq S Ahadi,
Shaun M Stracener,
Sherrie M La,
Sherry G Bous,
Shirley J Berryman,
Shwnji Susie Lee,
Sidney B Priesmeyer,
Simone E Pitts,
Sonia R Peterson,
Sony Mathews,
Sonya L Karsik, RAC,
Sparky L Bartee,
Sridhar Thumma,
Stacie A Woods,
Stephanie A Slater, MS,
Stephen J Koniers,
Stephen T Hansen,
Steve P Yost,
Steve Y Rhieu, PhD,
Steven A Gonzales,
Steven C Clifford,
Steven D Kehoe,
Steven P Allen,
Steven P Donald,
Stroman,
Sundy V Sedwick,
Sunita Masson,
Sunita Vij,
Susan F Laska, MS,
Susan M Jackson,
Susan T Hadman,
Susan W Ting,
Susanna E Ford,
Susanna N Choi,
Susie H Lee,
Suzanna E Ford,
Taichun Qin, PhD,
Tamala P Bogan,
Tamala P Magee,
Tamara J Umscheid,
Tamara L Setzer,
Tammy Vasquez Hancock,
Tara L Breckenridge,
Tara L Greene,
Tara L King,
Tara M Aikens,
Tawny L Colling,
Ted L Anderson,
Tenzin Jangchup,
Terrance L Thomas,
Terrance P Nguyen,
Terri L Dodds,
Thaddeus M Steinke,
Thai T Duong,
Thao T Kwan,
Theresa Kirkham (NMI),
Theressa B Smith,
Thomas J Arista,
Thomas R Beilke,
Thuy T Nguyen, LCDR,
Tiara Nbrown Crosen,
Timothy D Evans,
Timothy T Kapsala,
Tonia F Bernard,
Torrance J Slayton,
Torrey M Ward,
Toyin B Oladimeji,
Tracey L Harris,
Tracey T Duong,
Tracy K Li,
Travis S Bradley,
Tristian E Strait,
Trudy R Papson,
Truong Xuan Nguyen (Andy),
Trushani T Desai,
Uduak M Inokon,
Uttaniti Limchumroon (Tom),
Vaishali J Patel,
Vashti E Bocker,
Verdell Nelson,
Veronica Fuentes, MS,
Veronica L Call,
Vickie J Kanion,
Vickie L Anderson,
Vicky L Cruz,
Victoria A Wagoner,
Vinh T Phan,
Vioela J Caze,
Vipulchandra N Dholakia,
Virgilio F Pacio, CSO,
Virginia Carroll, PhD,
Virginia L Sheldon,
Walden H Lee,
Walsworth,
Warren J Lopicka,
Wayne D Mcgrath,
Wayne E Seifert,
Wenzheng Wendy Zhang,
Weston K Szymanski,
William J Leonard,
William S Vitale,
William V Millar,
Wr Bowman,
Xiaohui Shen,
Xiaokuang Lai, PhD,
Xiomara Copeland,
Yen Tso Kuo,
Yiyue Zhang (nmi), PhD,
Yumi J Hiramine,
Yvette E Guillermo,
Yvette Mlacour Davis,
Yvins Dezan,
Yvonne T Lacour,
Zachary L Miller,
Zachary L Stamm,
Zedong Dong,
Zhong Li, PhD,
Zhongren Wu
Zachary A Bogorad's Documents
Experience Redica Systems’ NEW investigator profiles and dashboards
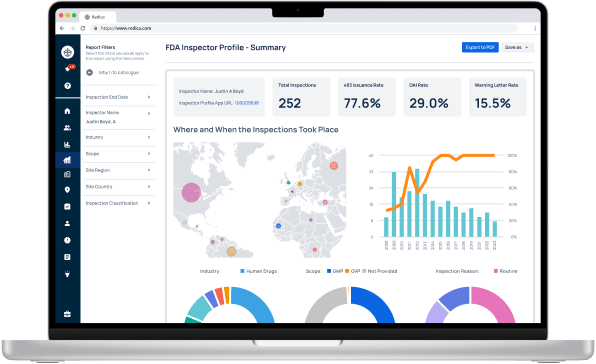
Redica Systems customers get access to over 1,400 FDA Investigator profiles, containing powerful analysis like:
- Total inspections conducted
- 483 rate
- OAI rate
- Warning Letter rate
- Inspections by Industry, Scope, and Inspection Reason
- And much more
Investigator Profiles are not sold as on-demand documents. If you’d like to see one, fill out the form below to connect with our Sales Team and get it in your inbox as little as 24 hours.
Talk to sales to access our investigator profiles
Loading...
Investigator Profiles Are Just the Tip of the Iceberg
Becoming a Redica Systems customer not only provides you with unlimited access to profiles similar to the one above for every current and former FDA Investigator, but also grants you access to:
Inspection Intelligence
- Pre-Approval Inspection (PAI) trends
- The ability to filter nearly any FDA enforcement action by GxP labels like GMP, GCP, etc.
- Latest 483s: see what the trends are across all FDA Investigators
- CFR Heatmap: Citations aggregated by Subpart by Year
Vendor Intelligence
- Full inspection histories for your vendors down to the specific site level
- All of the functionality mentioned above but isolated to just your vendors
Regulatory Intelligence
- Zero in on the trends for a specific agency/regulator (ex. FDA, MHRA, EMA, Health Canada)
- All the latest "Signals" from agencies around the world, segmented by Country, Type (ex. Guidance, Decree...), Category (ex. Rules/Regulations/Guidance, News, Reports...), Theme (ex. Cell and Gene Therapy, AI...), and more