FDA Investigator Byungja E Marciante
Byungja E Marciante has conducted inspections on 356 sites in 25 countries as of 27 May 2024. See below for a list of the FDA enforcement documents resulting from those inspections.
Investigator Details
Number of Inspected Sites:
356
Last Inspection Date:
27 May 2024
Investigator Role:
FDA Investigator
Redica ID:
Countries of Inspections:
United States of America,
China,
Puerto Rico,
Korea (Republic of),
Germany,
Serbia,
Italy,
Poland,
Sweden,
Austria,
Romania,
Japan,
Czechia,
New Zealand,
Greece,
Denmark,
Hong Kong,
Brazil,
Ireland,
Switzerland,
Russian Federation,
Thailand,
Australia,
United Kingdom of Great Britain and Northern Ireland,
Guam
FDA Investigators that have inspected at least one site in common with Byungja E Marciante:
Addam S Reynolds,
Albert M Brindle,
Alberto A Viciedo,
Alice S Tsao,
Allen Lou,
Alois P Provost,
Althea A Williams,
Amanda L Baskin,
Amy Franklin,
Amy L Singer,
Amy Lynn Frankshun, PhD,
Amy M Codella,
Amy M Cramer,
Amy S Graf,
Ana S Cabrera,
Anastasia I Offordile,
Anastasia I Onuorah,
Andrace L Deyampert,
Andrea A Branche,
Andrea D Swingle,
Andrew R Wasko,
Angel S Johnson,
Ann Marie Montemurro,
Ann Marie Schofield,
Anna R Kwilas,
Annemarie B Randow,
Annemarie Bodnar,
Annet R Rajan,
Anthony C Warchut,
Anthony J Donato,
Arindam Dasgupta, PhD,
Arthur S Williams, Jr,
Ashleigh P Barkans,
Ashleigh P Wodushek,
Atul J Agrawal,
Barbara D Wright,
Barbara J Holladay,
Barbara J Maulfair,
Barbara Janine Breithaupt,
Barbara Jwilimczyk Macri,
Barbara T Carmichael,
Bei Y He,
Bei Yu,
Benjamin J Smith,
Benton M Ketron,
Bernardica T Sculac Stern,
Bo Chi, PhD,
Brandy D Brown,
Brian D Garthwaite,
Brian D Young,
Brian J Mundo,
Brian M Campbell,
Brunilda Torres,
Burnell M Henry,
Carl J Montgomery,
Carmen Y Fisher,
Cary Greene,
Catherine J Laufmann,
CDR Ileana Barreto Pettit,
CDR Sean T Creighton,
Chaltu Nwakijra,
Charisse K Green,
Charles A Snipes, PhD,
Charles D Brown,
Charles I Ann,
Charles J Chacko,
Charles L Larson,
Charles L Zhou,
Charles M Edwards,
Charles R Bonapace, PhD,
Charles Yuanchia Kuo, PhD,
Charlotte Hinkle,
Cheryl A Grandinetti,
Cheryl D Mccall,
Cheryl J Legrand,
Chiang Syin, PhD,
Christian D Lynch (CDL),
Christina L Bigham,
Christina N Maurino,
Christina P Burkhart,
Christine E Kelley,
Christine I Shaw,
Christine M Cerenzio,
Christine M Whitby, CSO,
Christopher Janik,
Chryste D Best,
Chunsheng Cai, PhD,
Constantin Y Philopoulos,
Corrine M Carter,
Courtney R Bratina,
Craig A Garmendia,
Creighton T Tuzon,
Cynthia A Harris, MD, RN,
Cynthia F Kleppinger, MD,
Cynthia Jim, CSO,
Cynthia T Cain,
Cynthia Whitmarsh,
Daniel C Skirvin,
Daniel J Grabicki,
Daniel L Aisen,
Daniel R Azar,
Dannie E Rowland,
Darin S Wiegers,
David A Paterson,
David H Smith,
David L Duncan,
David P Vanhouten,
Dawn L Wydner,
Debara R Reese,
Debara Reese,
Deborah B Nixon,
Debra Bower,
Demitria J Argiropoulos,
Demitria J Xiradakis,
Dena M Elimam,
Denise L Burosh,
Denise M Visco, Investigator,
Denise M Visu,
Dennis E Guilfoyle, PhD,
Devon Jenkins,
Dhaval H Patel,
Diane C Thibodeau,
Diane M Gubernot,
Dianiris C Ayala,
Dien N Nguyen,
Dina A Tallman,
Dolores Harper,
Donald C Obenhuber, PhD,
Donna F Campbell,
Doreen P Canetti,
Doreen P Gubbay,
Dorothy J Denes,
Dorothy W Lee,
Douglas A Campbell,
Douglas C Kovacs,
Douglas Fiorentino Lcdr,
Dr. Abhijit Raha, PhD,
Dr. Mark J Seaton, PhD,
Dr. Robert C Horan, MD,
Dr. Sriram Subramaniam, PhD,
Dr. Zhou Chen (nmi), MD PhD,
Edmund F Mrak, Jr,
Edward D Mcdonald,
Edward J Janik,
Edward O'shaughnessy,
Elizabeth B Griffin,
Ellen P Madigan,
Emest F Bizjak,
Emily A Walters,
Emily J Orban,
Emmanuel Jramos Maldonado,
Eric A Mann,
Eric J Cunningham,
Eric Rothschild,
Eric S Pittman,
Erin D Mccaffery,
Erin L Mcfiren,
Esteban Beltran,
Ethan P Stegman,
Etty Feller,
Francis J Eng,
Frank J Marciniak,
Frederic W French, III,
Frederick F Razzaghi,
Gabrielle J Swain,
Gary J Hagan,
Gene D Arcy,
Gene R Gunn,
George G Calafactor,
George Pyramides,
George T Allen, Jr,
German Rivera,
Gianine E Tompkins,
Gifford Whitehurst, Jr,
Gillian B Nardelli,
Gobiga Vanniyasingam,
Gregson A Joseph,
Guerlain Ulysse,
Haley H Seymour,
Hang N Guo,
Helen B Ricalde,
Helen Verdel,
Hitesh R Patel,
Hugh M Mcclure, II,
Hyojong Kwon, PhD,
Iram R Hassan, PhD,
Irina Gaberman,
Iris C Macinnes,
J David Doleski,
Jacqueline A O'shaughnessy, PhD,
Jacqueline Mdiaz Albertini,
Jacqueline S Warner,
James A Beaulieu,
James D Planchon,
James E Frye,
James E Szelc,
James K Kane, PhD,
James R Birkenstamm,
Jane C Chen,
Janet Donnelly,
Jean Blackston Hill,
Jean M Kelahan,
Jean M Mulinde, MD,
Jeffrey A Sommers,
Jeffrey D Meng,
Jennifer A Bazergui,
Jennifer A Kemp,
Jennifer L Custodio,
Jennifer L Huntington,
Jennifer Macmillan,
Jesse A Vazquez,
Jessica M Monteiro,
Joan A Loreng,
Joan T Briones,
Joanne M Schlossin,
Joanne Pryzbylik,
Jocelyn C Turner,
Joel D Martinez,
John A Sciacchitano,
John B Crowe,
John Dan,
John P Mistler,
Jonah S Ufferfilge,
Jonathan G Matrisciano,
Jonathan Ho,
Jonee J Mearns,
Jorge F Christian,
Jorge L Guadalupe,
Joris Vanloco,
Jose M Cayuela,
Joseph A Piechocki,
Joseph F Mcginnis, RPh,
Joseph L Despins, PhD,
Joseph R Strelnik,
Joshua J Silvestri,
Joy Rkozlowski Klena,
Jude C Dikes,
Julie D Bringger,
Justin A Boyd,
Justine M Corson,
Justine Tomasso,
Jyoti B Patel, PhD,
K Gonzlez,
Kara A Scheibner, PhD,
Karen E D'orazio,
Karen F Tomaziefski,
Karen M Montgomery (KMM),
Kassa Ayalew, MD,
Kathleen B Swat,
Keith M Reilly,
Kelli F Dobilas,
Kellia N Hicks,
Kelly I Anderson,
Kelvin Cheung,
Kenneth Nieves,
Kenneth S Boehnen,
Kent A Conforti,
Kerun Hardeo,
Kimberly L Schultz,
Kimberly M Bigler,
Kinh Q Mac,
Kirtida Patel,
Kristen E Rescigno,
Kristina L Conroy,
Kristy A Zielny,
Krystal O Ogunremi,
Kurt A Brorson, PhD,
Kwong P Lee,
L'oreal F Walker,
Ladislav Kermet,
Lakecha N Lewis,
Lakisha M Williams,
Larry K Austin,
Lata C Mathew, PhD,
Laureen M Geniusz,
Lauren Iacono Connors, PhD,
Lauren L Vajo,
Lauren M Lawrance,
Lauren M Mclaughlin,
Laurie B Frazier,
LCDR Anastasia M Piliafas Brown,
LCDR Angelica M Chica,
LCDR Debra Emerson,
LCDR Jennifer H Rhyu,
LCDR Matthew R Mcnew, MPH, REHS/RS,
LCDR Randall L Morris,
LCDR Wilfred A Darang,
Leigh Anne Myers,
Lenahan Lenahan, Peter R,
Leo J Lagrotte,
Leon L Crawley,
Leonard H Lavi,
Li Li,
Li Yun Huang,
Liatte Kreuger, PharmD,
Linda A Gregory Duty,
Linda Thai,
Lisa Harlan,
Lisa M Bellows,
Lisa M Feola,
Loretta Nemchik,
Lori A Gioia,
Lori S Lawless,
LT Colin E Tack, BSE,
Luella J Rossi,
Lynda L Lanning,
Lynette P Salisbury,
Marcelo O Mangalindan, Jr,
Marea K Harmon,
Margaret M Sands,
Maria A Bienkowski,
Maria A Reed,
Maria Estrella,
Marie B Buen Bigornia,
Marijo B Kambere, PhD,
Marion Michaelis,
Mariza M Jafary,
Mark R Mcclain,
Marshall A Naimo,
Martha T Olone,
Martin K Yau, PhD,
Mary Jeanet Mcgarry,
Mary T Carden,
Maryam Tabatabaie,
Mathew T Thomas, MD,
Matthew A Spataro,
Matthew C Watson,
Matthew W Kyle,
Maya M Davis,
Mayar M Mussa,
Megan A Haggerty,
Mei Chen Mu,
Meihong Liu,
Meisha R Sampson,
Meisha Waters,
Melanie W Pishnery,
Melina Lrodriguez Upton,
Melissa A Freeman,
Melissa A Zuppe,
Melissa T Roy,
Melkamu Getie Kebtie, PhD,
Mercy Oyugi,
Meredith L Sheridan,
Meyer J Slobotsky,
Michael A Taylor,
Michael F Skelly, PhD,
Michael J Nerz,
Michael K Larson,
Michael P Anthony,
Michael P Sheehan,
Michael R Dominick,
Michael R Klapal,
Michael Rosner,
Michael S Kopf,
Michael Serrano,
Michael Shanks, MS,
Michael Skelly,
Michael Y Philopoulos,
Michele L Forster, PhD,
Michele L Obert,
Michelle A Marsh,
Michelle J Hines,
Michelle M Noe Varga,
Michelle M Parisi,
Mihaly S Ligmond,
Mike M Rashti,
Mishelle L Harriger,
Mohsen Rajabi Abhari, FDA,
Monika Borkowska,
Montgomery Montgomery, Karen M,
Msdap Gonzlezk,
Muna Algharibeh,
Myra K Casey,
Naakesh N Gomanie,
Nailing Zhang,
Namita Kothary,
Nancy F Scheraga,
Nancy G Schmidt,
Nancy L Rolli,
Nancy M Espinal,
Neali H Lucas,
Nealie C Newberger,
Nebil A Oumer,
Nerizza B Dalena,
Nerizza B Guerin,
Nicholas A Violand,
Nimmy Mathews,
Nina Yang,
Niraj R Mehta, PhD,
Norman K Starks,
Norman Wong,
Omotunde O Osunsanmi,
Patricia A Cochran,
Patricia H Dlugosz,
Patrick B Cummings,
Patrick D Stone, MS,
Patsy J Domingo,
Paul E Stein,
Paul L Bellamy,
Paul Mouris,
Paula A Trost,
Perry H Gambrell,
Peter R Lenahan,
Philip J Snoy,
Prabhu P Raju,
Preston M Lee,
Qiao Y Bobo,
Rachael O Oyewole,
Rachel C Harrington,
Rachel T Evans,
Rafeeq A Habeeb,
Raffi B Papazian,
Ralph A Erickson,
Ramon E Martinez,
Rapti D Madurawe,
Raymond L Cheung,
Raymond T Oji,
Rebecca E Dombrowski,
Rebecca Rodriguez,
Regina T Brown,
Remache,
Richard A Abate,
Richard D Manney,
Richard Heath Coats,
Richard Jay Tucker, RS,
Richard K Vogel,
Richard L Rutherford,
Robert C Steyert,
Robert D Tollefsen,
Robert G Ruff,
Robert J Ham,
Robert J Martin,
Robert Jennings,
Robert L Hummel,
Robert M Barbosa,
Robert O'brian,
Roberto A Dookhan,
Rodney B Ross,
Rodney D Combs,
Rodney T Allnutt,
Rory K Geyer,
Rose Ashley,
Russell J Glapion,
Ruth A Williams,
Sabine Francke,
Sam Chan,
Sandra A Boyd,
Sandra L Shire, DMD, MPA,
Sarah A Hassas,
Sarah Forney,
Sargum C Sood,
Satheesh Thomas,
Schultz,
Scott C Lute,
Scott T Ballard,
Sheri L Stephenson,
Sherri J Liu,
Sheryl A Kochman,
Shirley H Isbill,
Shirley S Wen,
Sidney B Priesmeyer,
Simone E Pitts,
Sinai I Davis,
Stacey S Degarmo,
Stephanie L Shapley,
Stephanie Mangigian, MS/OSH, RN,
Stephanie T Durso,
Stephen C Smith,
Stephen D Eich,
Stephen R Souza,
Steven P Donald,
Steven R Ziser,
Susan F Laska, MS,
Susan M Jackson,
Susan P Bruederle,
Susanne M Richardson, MS RAC,
Suyang Qin,
Talmane J Fisher,
Tamara S Rosbury,
Tammy Vasquez Hancock,
Tania E Garcia,
Tania E Vizcaino,
Tara G Bizjak,
Tara K Carmody,
Tara R Gooen,
Tawny L Colling,
Teresita C Mercado,
Terry C Kimball,
Thai T Duong,
Theressa B Smith,
Thomas J Arista,
Thomas P Hansen,
Thuy T Nguyen, LCDR,
Tiffani D Wilson,
Timothy C Schell,
Timothy T Kapsala,
Tina M Pawlowski,
Tonia F Bernard,
Tonia L Sawyer,
Tony T Yang,
Toyin B Oladimeji,
Tracey L Siebesma,
Tyanna N Hadley,
Uduak M Inokon,
Unnee Ranjan,
Valerie C Reed,
Vickie J Kanion,
Victoria M Daddeo,
Victoria Spivak,
Vincent F Cafiso,
Vivian Garcia,
Wayne E Seifert,
Wayne J Meyer,
William D Tingley,
William G Nelson,
William J Muszynski,
William R Chang,
William Regnault, PhD,
Xiaohan Cai, PhD,
Xiaoping Guan,
Xikui Chen (nmi), PhD,
Xingfang Li, MD,
Yangmin Ning,
Yumi J Hiramine,
Yvesna C Blaise,
Zakaria I Ganiyu,
Ze Peng,
Zhongren Wu
Byungja E Marciante's Documents
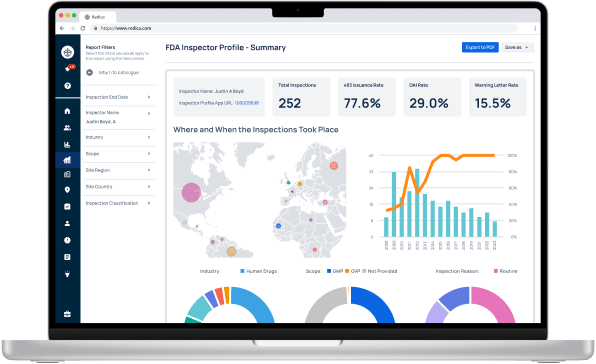
Experience Redica Systems’ NEW investigator profiles and dashboards
Redica Systems customers get access to over 1,400 FDA Investigator profiles, containing powerful analysis like:
- Total inspections conducted
- 483 rate
- OAI rate
- Warning Letter rate
- Inspections by Industry, Scope, and Inspection Reason
- And much more
Investigator Profiles are not sold as on-demand documents. If you’d like to see one, fill out the form below to connect with our Sales Team and get it in your inbox as little as 24 hours.
Talk to sales to access our investigator profiles
Loading...
Investigator Profiles Are Just the Tip of the Iceberg
Becoming a Redica Systems customer not only provides you with unlimited access to profiles similar to the one above for every current and former FDA Investigator, but also grants you access to:
Inspection Intelligence
- Pre-Approval Inspection (PAI) trends
- The ability to filter nearly any FDA enforcement action by GxP labels like GMP, GCP, etc.
- Latest 483s: see what the trends are across all FDA Investigators
- CFR Heatmap: Citations aggregated by Subpart by Year
Vendor Intelligence
- Full inspection histories for your vendors down to the specific site level
- All of the functionality mentioned above but isolated to just your vendors
Regulatory Intelligence
- Zero in on the trends for a specific agency/regulator (ex. FDA, MHRA, EMA, Health Canada)
- All the latest "Signals" from agencies around the world, segmented by Country, Type (ex. Guidance, Decree...), Category (ex. Rules/Regulations/Guidance, News, Reports...), Theme (ex. Cell and Gene Therapy, AI...), and more