FDA Investigator Ann Marie Montemurro
Ann Marie Montemurro has conducted inspections on 70 sites in 11 countries as of 03 May 2010. See below for a list of the FDA enforcement documents resulting from those inspections.
Investigator Details
Number of Inspected Sites:
70
Last Inspection Date:
03 May 2010
Investigator Role:
FDA Investigation Participant
Redica ID:
Countries of Inspections:
Canada,
United States of America,
Ireland,
United Kingdom of Great Britain and Northern Ireland,
Germany,
Denmark,
Italy,
France,
Switzerland,
Austria,
Belgium
FDA Investigators that have inspected at least one site in common with Ann Marie Montemurro:
Adetutu M Gidado,
Alan L Truong,
Albert A Salvador,
Alberto A Viciedo,
Alice C Silva,
Alicia Gilbert,
Alicia M Mozzachio,
Alla Kachko, PhD,
Allen Lou,
Alois P Provost,
Althea A Williams,
Amy L Singer,
Amy M Cramer,
Ana S Cabrera,
Anastasia G Lolas,
Anastasia I Offordile,
Anastasia M Shields,
Andrea Siegel, PhD,
Andrew C Chang,
Andria L Kuhlman,
Angela Jourdain,
Anissa M Cheung,
Anita Narula, PhD,
Anita R Michael,
Ann L Demarco,
Ann Marie Schofield,
Anne E Johnson,
Annemarie Bodnar,
Annette A Ragosta,
Anthony F Lorenzo,
Arduino Frankovic,
Arie C Menachem,
Arthur S Williams, Jr,
Ashley J Burns,
Atul J Agrawal,
Audrey Thereset Uy,
Barbara Janine Breithaupt,
Barbara Jwilimczyk Macri,
Barbara L Rellahan, PhD,
Barry Cherney, PhD,
Ben Du,
Bo Chi, PhD,
Brandon C Heitmeier,
Brandy N Lepage,
Brentley S Collins,
Brian D Nicholson,
Brian S Keefer,
Bruce D Meade,
Bruce H Mccullough,
Burnell M Henry,
Byungja E Marciante,
Calvin B Koerner,
Cara M Minelli,
Carl A Anderson,
Carl Lee,
Carla A Norris,
Carla J Lundi,
Carol L Rehkopf,
Carolyn A Renshaw,
Carolyn Yong, PhD,
Carrie Ann Plucinski,
Caryn M Mcnab,
Cassandra Overking,
CDR Donald Ertel,
CDR Jeremy L Wally, PhD,
Chad R Burger,
Chao Ming Tsai,
Charles I Ann,
Charles M Edwards,
Chiang Syin, PhD,
Christian D Lynch (CDL),
Christina A Miller,
Christina H Owens,
Christina J Sauder, PhD,
Christina K Theodorou,
Christina Theodorou,
Christina V Santos,
Christine A Harman, PhD,
Christopher J Adams,
Christopher R Czajka,
Claire M Minden,
Craig D Zagata,
Creighton T Tuzon,
Cynthia Jim, CSO,
Cynthia L Kelley,
Cynthia White,
Daniel J Grabicki,
Daniel Lagasse,
Daniel T Lee,
Dave Hafner,
David E Bailey,
David J Hafner,
David J Hapner,
David K Lau,
David M Beltran,
David Perkins,
Dawn L Wydner,
Deborah B Nixon,
Deborah M Trout,
Debra I Love,
Debra J Bennett,
Debra L Pagano,
Dennis Cantellops Paite,
Derek C Price,
Destry M Sillivan,
Dinesh Kumar,
Dino A Fiegelstock,
Donald C Obenhuber, PhD,
Dongping Dai, PhD,
Douglas C Kovacs,
Dr. Barbara D Paul, PhD,
Dr. Gang Wang, PhD,
Dr. Guang Gao, PhD,
Dr. Mark J Seaton, PhD,
Dr. Ralph M Bernstein, PhD,
Dr. Robert C Horan, MD,
Dr. Willie F Vann, PhD,
Dr. Willie Wilson, PhD,
Dr. Zhihao Qiu (Peter), PhD,
Dr. Zhou Chen (nmi), MD PhD,
Edmund F Mrak, Jr,
Edward Patten,
Eileen A Liu,
Eliezar Ramos,
Ellen Huang,
Ellen P Madigan,
Elmina E Akwo,
Emmanuel Jramos Maldonado,
Eric J Cunningham,
Eric Rothschild,
Erika M Wilkerson,
Erika V Butler,
Erin D Mccaffery,
Eural E Porter,
Frank J Marciniak,
Frank Wackes,
Freddy Ortiz Colon,
Frederick F Razzaghi,
Fulton A Varner,
Gabrielle J Swain,
Gayle S Lawson,
Gene D Arcy,
George Pyramides,
Gerald B Seaborn, Jr,
Gianine E Tompkins,
Ginger M Sykes,
Grace E Mcnally,
Guerlain Ulysse,
Gunther Boekhoudt, PhD,
Gwyn G Dickinson,
Haitao Li,
Hala L Selby,
Haruhiko Murata,
Helen B Ricalde,
Helen R Bester,
Helen Verdel,
Holly Brevig,
Humera T Khan,
Hyesuk Kong,
Ivis L Negron,
J David Doleski,
Jacqueline Mdiaz Albertini,
James C Lee, PharmD,
James C Maclaughlin,
James E Keller, PhD,
James L Dunnie, Jr,
James L Kenney, DSc,
James M Mason,
James M Simpson,
James P Mcevoy,
James R Evans,
James T Mccoy,
Janet L Bowen,
Jason F Chancey,
Jawaid Hamid,
Jean Lhu Primmer,
Jean M Kelahan,
Jeanne M Morris,
Jeffrey M Watson,
Jennifer L Bridgewater,
Jennifer L Schmidt,
Jennifer Macmillan,
Jessica L Pressley,
Jianming Li,
Jie He,
Joan A Loreng,
Joan Adamo,
Joan Johnson,
Joanne E King,
Joanne M Hajoway,
Joanne Pryzbylik,
Jocelyn C Turner,
Jogy George,
John M Mcinnis,
John S Hartford,
John Stansberry, PhD,
Jonathan G Matrisciano,
Jonee J Mearns,
Jose Acruz Gonzalez,
Jose M Cayuela,
Joseph F Mcginnis, RPh,
Joseph George,
Joseph L Despins, PhD,
Joseph R Lambert,
Joy Rkozlowski Klena,
Jude C Dikes,
Julianne C Mccullough,
Julie D Bringger,
Junho Pak,
Justin A Boyd,
Justine Tomasso,
Kalavati Suvarna, PhD,
Karen A Coleman,
Karen E D'orazio,
Karyn M Campbell,
Katherine E Jacobitz,
Kathleen B Mihalik,
Kathryn Carbone, MD,
Kelli F Dobilas,
Kelly I Anderson,
Kelly N Kerr,
Kelvin Cheung,
Kendra L Brooks,
Kenneth M Gordon,
Kent C Faul,
Kevin D Kallander,
Kevin M Maguire,
Kevin P Foley,
Kim Lthomas Cruse,
Kimberley A Hoefen,
Kimberly M Bigler,
Kinh Q Mac,
Kristen D Evans,
Kristina J Donohue,
Kristina Mjoyce Pittman,
Kristy A Zielny,
Kurt A Brorson, PhD,
Lakshmi Narasimhan, PhD,
Larry K Austin,
Laura Fontan, MS,
Laurel A Beer,
Lauren L Vajo,
Laurie B Frazier,
Laurie P Norwood,
LCDR Debra Emerson,
LCDR Margaret Edi Gennaro,
LCDR Susan E Polifko,
Lequita M Mayhew,
Leslie D Wagner,
Li Li,
Liatte Kreuger, PharmD,
Linda Thai,
Lindan E Silvers,
Lindsey R Lyman,
Lisa M Bellows,
Lisa M Feola,
Lisa P Oakes,
Lixia Cai,
Lorelei S Jarrell,
Loretta Nemchik,
Lori S Lawless,
LT John M Mastalski,
LTJG Bradley E Benasutti,
Lu Deng,
Lydia I Rosas Marty,
Madushini Dharmasena, PhD,
Maotang Zhou, PhD,
Marc S Neubauer,
Marcelo O Mangalindan, Jr,
Marcus F Yambot,
Margaret M Annes,
Maria Estrella,
Marian E Major, PhD,
Marie B Buen Bigornia,
Marie F Morin,
Marion Michaelis,
Mariza M Jafary,
Mark R Mcclain,
Marla A Cassidy,
Marlene G Swider,
Marsha W Major,
Martha O'lone,
Martha Sullivan Myrick,
Martha T Olone,
Mary P Padgett,
Mary T Carden,
Maryna C Eichelberger, PhD,
Matthew A Spataro,
Maxwell Van Tassell, PhD,
Megan A Haggerty,
Meihong Liu,
Melba Trivera Clavell,
Melissa D Ray,
Melissa T Roy,
Michael A Charles,
Michael Curbarg,
Michael D Omeara,
Michael Gurbarg,
Michael R Goga,
Michael R Klapal,
Michael Schmitt,
Michael Serrano,
Michael Shanks, MS,
Michele L Forster, PhD,
Michele Perry Williams,
Mihaly S Ligmond,
Mike M Rashti,
Mikhail V Ovanesov, PhD,
Mindy M Chou,
Minh D Phan,
Mizanne E Lewis,
Morris Paul,
Morris Sheldon, PhD,
Mra Davism,
Mra M Barbosar,
Mra Mcculloughj,
Murthy Vkn Sikha,
Myra K Casey,
Nancy E Kirschbaum,
Nancy F Scheraga,
Nancy G Schmidt,
Nancy L Neiger,
Nancy L Rolli,
Nancy T Waites,
Nawab A Siddiqui,
Nerizza B Guerin,
Nicholas A Violand,
Nicholas F Lyons,
Nicholas P Diorio,
Nicole J Clausen,
Nicole K Trudel,
Niketa Patel,
Nikisha M Bolden,
Nimmy Mathews,
Niraj R Mehta, PhD,
Nisha C Patel,
Noreen Muñiz,
Omotunde O Osunsanmi,
Pankaj H Amin,
Parul M Patel,
Patricia A Morrow,
Patricia F Hudson,
Patricia F Hughes, PhD,
Patrick C Klotzbuecher,
Patsy J Domingo,
Paul A Bonneau,
Paul E Stein,
Paul L Bellamy,
Paul W Moy,
Paul Z Balcer,
Paula A Trost,
Peter R Lenahan,
Philip F Istafanos, DMV, MS,
Phillip M Pontikos,
Phung Thien Nguyen,
Prabhu P Raju,
Priscilla M Pastrana,
Qiao Y Bobo,
Rajiv R Srivastava,
Ralph A Erickson,
Ralph Jerndal,
Ramesh Potla, PhD,
Ramjay Vatsan, PhD,
Ramon E Martinez,
Randa Melhem, PhD,
Rebecca K Olin,
Rebecca Rodriguez,
Regina Gibson Melton,
Regina T Brown,
Reginald D Neal,
Reyes Candau Chacon, PhD,
Richard D Manney,
Richard H Penta,
Richard Jay Tucker, RS,
Richard K Vogel,
Richard Ledwidge (nmi), PhD,
Richard T Riggie,
Richard W Thornton,
Robert B Shibuya, MD,
Robert C Coleman,
Robert D Tollefsen,
Robert J Maffei,
Robert J Martin,
Robert Jennings,
Robert L Stevenson,
Robert Sausville,
Robin P Mathew,
Rochelle K Kimmel,
Rodney D Combs,
Roger F Zabinski,
Roman T Drews, PhD,
Rona A Leblanc, PhD,
Rona Leblanc, PhD,
Ronald T Nowalk,
Rory K Geyer,
Rose Ashley,
Rumany C Penn, PharmD,
Russ R Rivy,
Russell J Glapion,
Russell K Riley,
Samantha J Bradley,
Samuel K Gibbons, Jr,
Sandra Kershaw,
Sarah Adella Fave,
Sarah B Tanksley,
Sarah Forney,
Scott E Norris,
Scott T Ballard,
Sean R Marcsisin,
Sergey S Akimov,
Sharon K Thoma, PharmD,
Sheila Dreher Lesnick,
Sheldon Morris,
Shelley L Waring,
Sherry G Bous,
Sheryl A Kochman,
Shirley H Isbill,
Shuang Tang,
Shwnji Susie Lee,
Sidney B Priesmeyer,
Simone E Pitts,
Sinai I Davis,
Solomon Yimam (SY),
Stephanie A Slater, MS,
Stephanie Mangigian, MS/OSH, RN,
Stephen C Smith,
Stephen D Brown,
Stephen D Eich,
Steven A Gonzales,
Steven A Rubin,
Steven C Derrick,
Steven C Madzo,
Steven E Bowen, PhD,
Steven L Carter,
Steven M Weinman,
Steven P Donald,
Subramanian Muthukkumar, PhD,
Susan A Zullo, PhD,
Susan F Laska, MS,
Susan M Jackson,
Susan P Bruederle,
Susan T Hadman,
Susan Yu,
Tamil Arasu, PhD,
Tammy L Chavis,
Tania E Vizcaino,
Temar Q Williams,
Teresita C Mercado,
Terrig Thomas, PhD,
Thai D Truong,
Thomas E Friel,
Thomas J Arista,
Thomas R Withers,
Thunder N Dunkijacobs,
Thuy T Nguyen, LCDR,
Tina S Roecklein,
Todd J Maushart,
Todd M Stankewicz,
Troy C Quander,
Unnee Ranjan,
Verlinda A Narcisse,
Vijaya L Simhadri,
Virgilio F Pacio, CSO,
Viviana Matta,
Vlada Matusovsky,
Warren J Lopicka,
Wayne E Seifert,
Wayne J Meyer,
Wayne T Smith,
Wei Wang, PhD,
William D Tingley,
Xiao Wang,
Xiaokuang Lai, PhD,
Yangmin Ning,
Yanming An, PhD,
Yasamin Ameri,
Yehualashet A Gessesse,
Yonggang Wang, PhD,
Yumi J Hiramine,
Zachary L Miller,
Zachary L Stamm,
Zakaria I Ganiyu,
Zhijin Chen,
Zhong Li, PhD,
Zuben E Sauna, PhD
Ann Marie Montemurro's Documents
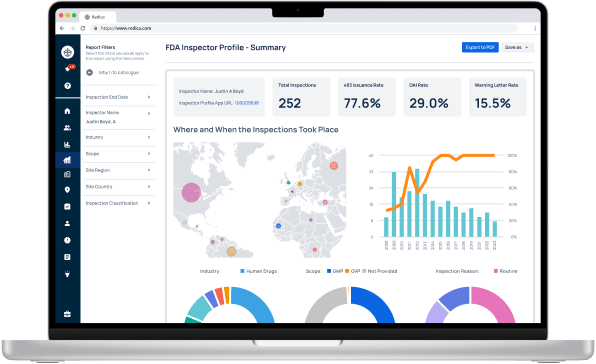
Experience Redica Systems’ NEW investigator profiles and dashboards
Redica Systems customers get access to over 1,400 FDA Investigator profiles, containing powerful analysis like:
- Total inspections conducted
- 483 rate
- OAI rate
- Warning Letter rate
- Inspections by Industry, Scope, and Inspection Reason
- And much more
Investigator Profiles are not sold as on-demand documents. If you’d like to see one, fill out the form below to connect with our Sales Team and get it in your inbox as little as 24 hours.
Talk to sales to access our investigator profiles
Loading...
Investigator Profiles Are Just the Tip of the Iceberg
Becoming a Redica Systems customer not only provides you with unlimited access to profiles similar to the one above for every current and former FDA Investigator, but also grants you access to:
Inspection Intelligence
- Pre-Approval Inspection (PAI) trends
- The ability to filter nearly any FDA enforcement action by GxP labels like GMP, GCP, etc.
- Latest 483s: see what the trends are across all FDA Investigators
- CFR Heatmap: Citations aggregated by Subpart by Year
Vendor Intelligence
- Full inspection histories for your vendors down to the specific site level
- All of the functionality mentioned above but isolated to just your vendors
Regulatory Intelligence
- Zero in on the trends for a specific agency/regulator (ex. FDA, MHRA, EMA, Health Canada)
- All the latest "Signals" from agencies around the world, segmented by Country, Type (ex. Guidance, Decree...), Category (ex. Rules/Regulations/Guidance, News, Reports...), Theme (ex. Cell and Gene Therapy, AI...), and more