FDA Investigator Barbara J Rincon
Barbara J Rincon has conducted inspections on 497 sites in 18 countries as of 13 Nov 2018. See below for a list of the FDA enforcement documents resulting from those inspections.
Investigator Details
Number of Inspected Sites:
497
Last Inspection Date:
13 Nov 2018
Investigator Role:
FDA Investigator
Redica ID:
Countries of Inspections:
United States of America,
France,
Switzerland,
Czechia,
Georgia,
Australia,
United Kingdom of Great Britain and Northern Ireland,
China,
Japan,
Poland,
India,
Italy,
Germany,
Korea (Republic of),
Russian Federation,
Croatia,
Netherlands,
Belgium
FDA Investigators that have inspected at least one site in common with Barbara J Rincon:
Aaron J Poloni,
Abby M Miller,
Ademola O Daramola,
Alexander M Kay,
Alexandra B Pitkin,
Alicia Aborg Borm,
Amber M Howell,
Amy M Cramer,
Amy R Glynn,
Anastasia M Shields,
Andrew T Todd,
Angela Shepas,
Anissa M Vargas,
Anita Narula, PhD,
Anna J Vickrey,
Anna Lazar,
Annette L Diggs,
Anthony A Charity,
Antonetta M Colacchio,
April P Shaw,
Arie R Dyk,
Ariel Cruz Figueroa,
Arnold Seo, PhD,
Ashleigh P Barkans,
Astrida B Mattson,
Atul J Agrawal,
Azza Talaat,
Babajide Michael Osunsanmi,
Barbara Jwilimczyk Macri,
Barbara M Frazier,
Beau R Lamb,
Belinda E Clifton,
Bichsa T Tran,
Binh T Nguyen,
Brandy N Lepage,
Brian D Nicholson,
Brian P Putz,
Bruce R Burrell,
Bryce A May,
Burnell M Henry,
Carla J Lundi,
Carrie A Hughes,
Caryn M Mcnab,
Cathleen A Carr Sharpe,
Celena Ngo,
Celia L Hutchinstein,
Celia L Silverstein,
Charles I Ann,
Charles L Larson,
Charles M Edwards,
Charles R Cote, RIC,
Cheryl D Mccall,
Christina K Theodorou,
Christopher Do,
Christopher R Czajka,
Christopher S Genther,
Clint R Van Blaricom,
Comyar Shoghi,
Constance Y Fears,
Crystal A Harlan,
Crystal Monroy,
Crystal S Harris,
Cynthia A Palmer,
Dale A Nyberg,
Daniel A Congdon,
Daniel J Gorski,
Daniel W Cline,
Danny D Horner,
Darren S Brown,
David M Beltran,
David P Moorhead,
Davis,
Dawn E Barkans,
Dawn Smith,
Deborah A Greco,
Deborah A Nebenzahl,
Debra C Yamane,
Dejon N Harris,
Demitria J Xiradakis,
Dennis Cantellops Paite,
Dennis G Kawabata,
Dennis M Farley,
Dennis R Hudson,
Derek S Dealy,
Desiree C Iya,
Devon M Shoop,
Diana L Ayala,
Diana M Rand,
Dirk L Lincoln,
Don A Brunssen,
Donald B Mckechnie,
Donna Ltartaglino Besone,
Dorine J Hagstrom,
Dr. Mark J Seaton, PhD,
Dr. Zhou Chen (nmi), MD PhD,
Edmund F Mrak, Jr,
Elizabeth A Sheller,
Elizabeth S Howell,
Emal Wahab,
Emmanuel B Diric,
Eric L Scott,
Erica R Mahone,
Evelyn Taha,
Farhana Khan,
Felix Maldonado,
Francis J Eng,
Francisco,
Gail E Flory,
Gajendiran Mahadevan, PhD,
Gale E Ryan,
Garciam,
Gavin W Pierce,
Gerald B Seaborn, Jr,
Gerard Pde Leon,
Ginger M Sykes,
Gloria J Baca, MS,
Graham N Giesen,
Gulshan R Anand,
Gwyn G Dickinson,
Hamet M Toure, PharmD MPH,
Harry J Brewer,
Heriberto Negron Rivera,
Himanshu Gupta, PhD,
Hugh A Grimoldby,
Hughes,
Ian J Thomson,
Isaiah D Isakson,
Ivis L Negron,
Jack L Christy,
Jacob W Reynolds,
Jacobitz,
James A Barreto,
James C Maclaughlin,
James D Hildreth,
James E Trabert,
James P Stallings,
James R Montero,
James S Cartwright,
Jane S Wernberg,
Janete F Guardia,
Jason W Cornell,
Jawaid Hamid,
Jean Brewer,
Jean C Colon Lopez,
Jean R Mccurry,
Jeanne M Weishaar,
Jeannine K Wooten,
Jeff A Gard,
Jeffrey J Leclair,
Jeffrey N Gerdes,
Jennifer C Adams,
Jennifer L Huntington,
Jennifer M Gogley,
Jessica B Clark,
Jessica L Pressley,
Jiao Yang, PhD,
Joan M Cantellops Figueroa,
Joanne M Schlossin,
Jocelyn E Massey,
Joel D Hustedt,
Joey V Quitania,
John A Daubenspeck,
John A Gonzalez,
John C Sedwick,
John D White,
John E Emmert,
Jolanna A Norton,
Jonathan T Little,
Jonetta I Collins,
Jorge M Ruano Rossil,
Jose A Lopez,
Jose Acruz Gonzalez,
José E Meléndez,
Jose M Cayuela,
Joseph A Seitz, III,
Joseph M Willems,
Joseph R Haynes,
Judy C Nepsa,
Julia Ventura,
Julian C Hanson,
Junho Pak,
Karen A Briggs,
Karl D Hezel,
Karyn M Campbell,
Katharine Chan,
Katherine E Jacobitz,
Katherine L Arnold,
Kathleen B Swat,
Keishla M Arroyo Lopez,
Keith C Littrell,
Kellia N Hicks,
Kelly D Moore,
Kelly D Sheppard,
Kelly S Mammen,
Kelsey A Hustedt,
Kelsey A Volkman,
Kelsey M Bishop,
Kelvin X Sanders,
Kenneth O Gee, PhD,
Kent C Faul,
Kevin T Gerrity,
Kham Phommachanh,
Khang K Pham,
Khanh T Van,
Khoa Nathanv Tran,
Kim Lthomas Cruse,
Kimberly Jproctor Jones,
Kimberly M Lichter,
Kristi B Panzer,
Kristina M Phelps,
Kristy A Zielny,
Kurt M Hafer,
L Grant,
Lakecha N Lewis,
Lam,
Lance W Bohm,
Lauren L Vajo,
Lawrence Y Lee, PhD,
LCDR Ismael Olvera, IV,
Leon R Marquart,
Lesley A Swanson,
Lester O Boian,
Ligia M Cline,
Linda Thai,
Ling Yu L Liu,
Lisa A Bowden,
Lisa K Capron,
Lisa M Lopez,
Lon A Cummings,
Lopez,
Loretta J Jordan,
Lori J Jennings,
Lucas B Leake,
Luis A Dasta,
Madushini Dharmasena, PhD,
Mai Hang,
Mai X Huynh,
Marc A Jackson, Jr,
Marcellinus D Dordunoo,
Marcus A Goshen,
Marcus F Yambot,
Margo C Jones,
Mark C Saale,
Mark E Imsland,
Mark L Collins,
Mark R Mcclain,
Marsha L Mccauley,
Massoud Motamed,
Matthew B Casale,
Maurice M Sheehan,
Megan A Haggerty,
Mei Chen Mu,
Michael A Charles,
Michael D Garcia,
Michael F Schuette,
Michael J Lackey,
Michael S Hudson,
Michele R Douglas,
Miguel A Martinez Perez,
Mikel T Wright,
Minh D Phan,
Misty L Wallace,
Mra B Kamberem,
Mra Greenc,
Mra Mcculloughj,
Myers,
Nadeem I Chaudhry,
Nancy A Saxenian Emmons,
Nancy E Byerly,
Nancy E Doyle,
Nancy G Schmidt,
Narahi J Alvarez Alcazar,
Natalie J Ayoub,
Neil J Traaen,
Ngoc Lan T Nguyen,
Nianna C Burns,
Nicholas L Hunt,
Pamela Y Lee,
Parul M Patel,
Patricia H Dlugosz,
Patrick C Klotzbuecher,
Patty P Kaewussdangkul,
Paul E Frazier,
Paul F Rader,
Paul M Kawamoto,
Paula J Wilkerson,
Peter Kessler, PhD,
Peter T Regan,
Phal K Chhun,
Philippe A Cornet,
Phillip Angart, PhD,
Phillip L Toy,
Rafael E Arroyo,
Randall N Johnson,
Rebecca T Davis,
Richard T Jensen,
Richard W Tubb,
Richmond K Yip,
Robert C Coleman,
Robert D Tollefsen,
Robert J Martin,
Robert J Vezzetti,
Robert J Waldorf,
Rocco C Black,
Rodrigo O Chipres,
Roger F Zabinski,
Roger J Adams,
Rose Ljean Mary,
Roy R Rinc,
Rumany C Penn, PharmD,
Ruth A Hoskins,
Ryan J Borges,
Saied A Asbagh,
Sandra A Boyd,
Sangeeta M Khurana, PhD,
Sara M Onyango,
Sarah E Mcmullen,
Sarah M Meng,
Sarah M Napier,
Scott A Nabe,
Scott J Matsuda,
Scott N Lim,
Scott W Fox,
Sean P Desbrow,
Sena G Dissmeyer,
Shane R Teeter,
Shannon J Hall,
Sharon K Thoma, PharmD,
Shelagh D Schopen,
Shelly M King,
Sherri N Rohlf, MD,
Shirley J Berryman,
Simone E Pitts,
Sonia R Peterson,
Sony Mathews,
Sripal R Mada, PhD,
Stacy A Schneider,
Stanley B Eugene, BS, BME,
Stephanie A Jameson,
Stephanie A Slater, MS,
Stephanie L Shapley,
Stephanie Mangigian, MS/OSH, RN,
Stephen R Souza,
Steve Wong,
Steven E Porter, Jr,
Sundy V Sedwick,
Sunitha K Rajaram, PhD,
Susan F Laska, MS,
Susan K Bain,
Susan Kendrick,
Susan T Hadman,
Susan W Ting,
Suyang Qin,
Taichun Qin, PhD,
Tamala P Bogan,
Tamala P Magee,
Tamara L Ely,
Tara M Pianko,
Tawny L Colling,
Teena H Aiken,
Terri L Dodds,
Terry C Kimball,
Thanh M Andrews,
Thao T Kwan,
Thomas C Mclean, CFS,
Thomas J Arista,
Thomas J Scott,
Thomas R Beilke,
Thomas W Gordon,
Timothy P Lafave,
Timothy T Kapsala,
Toby Vern H Hill,
Tonia L Sawyer,
Tracy K Li,
Truong Xuan Nguyen (Andy),
Trushani T Desai,
Uttaniti Limchumroon (Tom),
Valerie L Whipp,
Vilmary Negron Rodriguez,
Vitaliy N Guyvan,
Viviana Matta,
Walden H Lee,
Wayne D Stowell,
Willemsj,
William A Burkey,
William C Hughes,
Yasamin Ameri,
Yumi J Hiramine,
Zachary A Bogorad,
Zachary P Wachlarowicz,
Zerita White
Barbara J Rincon's Documents
| Publish Date | Document Type | Title |
|---|---|---|
| January, 2016 | FDA 483 | Hainan Poly Pharm. Co., Ltd. - Form 483, 2016-01-29 |
| August, 2014 | FDA 483 | Herbs America, Inc. - Form 483, 2014-08-25 |
| April, 2000 | EIR | Constant Irwindale Inc. - EIR, 2000-04-28 |
| July, 2000 | EIR | Pet Center Inc - EIR, 2000-07-06 |
| April, 2000 | FDA 483 | Anthony Products - Form 483, 2000-04-28 |
| August, 2014 | EIR | Herb Pharm, LLC - EIR, 2014-08-28 |
| July, 2012 | FDA 483 | SciCan GmbH BHT - Form 483, 2012-07-19 |
| September, 2010 | FDA 483 | Zorro Technology, Inc. - Form 483, 2010-09-14 |
| October, 2013 | FDA 483 | Frantisek Tousek - Form 483, 2013-10-25 |
| September, 2016 | FDA 483 | Shionogi Pharma Co., Ltd. - Form 483, 2016-09-16 |
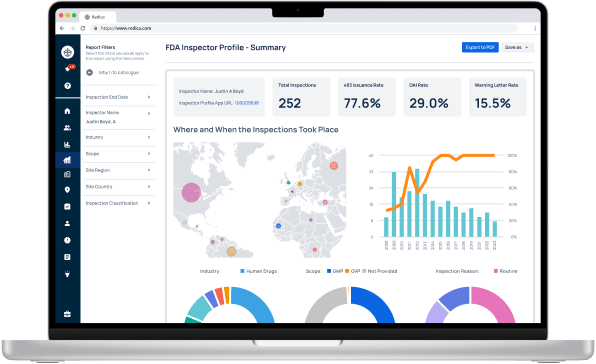
Experience Redica Systems’ NEW investigator profiles and dashboards
Redica Systems customers get access to over 1,400 FDA Investigator profiles, containing powerful analysis like:
- Total inspections conducted
- 483 rate
- OAI rate
- Warning Letter rate
- Inspections by Industry, Scope, and Inspection Reason
- And much more
Investigator Profiles are not sold as on-demand documents. If you’d like to see one, fill out the form below to connect with our Sales Team and get it in your inbox as little as 24 hours.
Talk to sales to access our investigator profiles
Loading...
Investigator Profiles Are Just the Tip of the Iceberg
Becoming a Redica Systems customer not only provides you with unlimited access to profiles similar to the one above for every current and former FDA Investigator, but also grants you access to:
Inspection Intelligence
- Pre-Approval Inspection (PAI) trends
- The ability to filter nearly any FDA enforcement action by GxP labels like GMP, GCP, etc.
- Latest 483s: see what the trends are across all FDA Investigators
- CFR Heatmap: Citations aggregated by Subpart by Year
Vendor Intelligence
- Full inspection histories for your vendors down to the specific site level
- All of the functionality mentioned above but isolated to just your vendors
Regulatory Intelligence
- Zero in on the trends for a specific agency/regulator (ex. FDA, MHRA, EMA, Health Canada)
- All the latest "Signals" from agencies around the world, segmented by Country, Type (ex. Guidance, Decree...), Category (ex. Rules/Regulations/Guidance, News, Reports...), Theme (ex. Cell and Gene Therapy, AI...), and more