FDA Investigator Jessica L Pressley
Jessica L Pressley has conducted inspections on 155 sites in 17 countries as of 23 Aug 2021. See below for a list of the FDA enforcement documents resulting from those inspections.
Investigator Details
Number of Inspected Sites:
155
Last Inspection Date:
23 Aug 2021
Investigator Role:
FDA Investigator
Redica ID:
Countries of Inspections:
United States of America,
Germany,
Taiwan,
Canada,
Switzerland,
Finland,
Malta,
Ireland,
United Kingdom of Great Britain and Northern Ireland,
Hong Kong,
India,
Japan,
Denmark,
China,
Italy,
Macao,
Mexico
FDA Investigators that have inspected at least one site in common with Jessica L Pressley:
Abdollah Koolivand,
Ademola O Daramola,
Alan L Truong,
Albert A Salvador,
Alice S Tsao,
Althea A Williams,
Ana Delp Cintron,
Ana P Barido,
Ana P Pineda Zavaleta,
Andrea A Branche,
Andrea H Norwood,
Andrew J Mcguffin,
Angela E Glenn,
Ann M Stewart,
Ann Marie Montemurro,
Anthony F Lorenzo,
Ashley A Mutawakkil,
Ashley A Segura,
Ashley B Jelonek,
Ashley Gonzalez,
Azza Talaat,
Barbara J Rincon,
Bernice F Hirsch,
Betsy C Galliher,
Bichsa T Tran,
Bill Tacket, Jr,
Blanchie M Speights,
Bonita S Chester,
Brandi L Garbutt,
Brandon C Heitmeier,
Brandy N Lepage,
Brant M Schroeder,
Brian R Yaun,
Brittny C Cargo,
Brooke K Higgins,
Brooke K Seeman,
Brunilda Torres,
Burnell M Henry,
Candice C Mandera,
Candice J Cortes,
Carla A Norris,
Carla J Lundi,
Caroline Strasinger,
Caryn M Mcnab,
CDR Ileana Barreto Pettit,
Charisse K Green,
Charles M Edwards,
Charles R Cote, RIC,
Charles S Watson,
Cheryl A Clausen,
Chiaochun J Wang,
Christian D Lynch (CDL),
Christie A Soto,
Christina K Theodorou,
Christos G Tsingelis,
Chyla T Hunter,
Claire M Minden,
Clifton L Randell,
Clinton J Lott,
Cody D Rickman,
Concepcion Cruz, Jr,
Constance Y Fears,
Courtney A Gilbert,
Courtney Hunt,
Craig A Garmendia,
Cynthia J Lee, MS,
Cynthia Jim, CSO,
Damaris Y Hernandez,
Dandan Wang, PhD,
Daryl A Dewoskin,
David A Oluwo,
David J Hafner,
David J Leray,
David P King,
Dawn M Mccabe,
Deborah A Greco,
Deborah A Racioppi,
Demario L Walls,
Desiree D Clark,
Deyaa Shaheen,
Diane L Kelsch,
Diane P Goyette,
Dillard H Woody, Jr,
Dionne D Arline,
Dipti Kalra,
Don H Bark, PhD,
Douglas A Campbell,
Dr. Chunchang Fang,
Dr. Dominick Roselle,
Dr. Gang Wang, PhD,
Dr. Ralph M Bernstein, PhD,
Dr. Zhihao Qiu (Peter), PhD,
Dusty F Snoeberg,
Dyvette Arline,
Edward D Mcdonald,
Edward H Maticka,
Elizabeth W Ormond,
Emest F Bizjak,
Emma R Schaefer,
Erika V Butler,
Erin D Mccaffery,
Ernest A Clausnitzer,
Ernest L Levins,
Ethan P Stegman,
Fabian Nchaparro Rodriguez,
Felix Maldonado,
Frank Wackes,
Gavin T Cua,
Gene R Gunn,
George J Flynn,
Gerald B Seaborn, Jr,
Gerard Pde Leon,
German Rivera,
Gideon N Esuzor,
Gina C Chen,
Gina Eng,
Gregory Price,
Gwyn G Dickinson,
Haijing Hu,
Haitao Li,
Haley H Seymour,
Harry R Bringger, Jr,
Harshal J Desai,
Helen Verdel,
Heriberto Negron Rivera,
Holly M Scott,
Israel Juarbe,
Ivis L Negron,
Jacqueline Mdiaz Albertini,
James C Maclaughlin,
Jamie L Port,
Jana L Caylor,
Jared P Stevens,
Jason D Tenney,
Jason K Morgan,
Jason P Aun,
Javier O Vega,
Jay T Wong,
Jennifer A Robinson,
Jennifer Caycedo,
Jennifer D Hollstrom,
Jennifer L Bridgewater,
Jennifer L Huntington,
Jennifer Lalama,
Jennifer M Menendez,
Jessica M Simpson, PhD,
Jessica P Mcalister,
Joanne E King,
Joey V Quitania,
Jogy George,
John B Crowe,
John W Banks,
Jonathan W Chapman,
Jose A Lopez,
Jose Acruz Gonzalez,
Jose M Cayuela,
Jose M Gonzalez,
Jose Ohernandez Guzman,
Jose Perez Soto,
Jose R Hernandez,
Jose R Rodriguez,
Jose Velez,
Joseph J Vannelli,
Joseph R Lambert,
Joshua J Silvestri,
Joshua P Wireman,
Juan C Gonzalez Rivera,
June P Page,
Justin A Boyd,
Karen E D'orazio,
Karen K Moksnes,
Karen Takahashi,
Karissa J Mccaw,
Karl D Hezel,
Katherine E Jacobitz,
Katherine Szestypalow,
Kathleen M Sinninger,
Kayla V Sprague,
Kelly I Anderson,
Kelly L Anderson,
Kemejumaka N Opara,
Kenneth Nieves,
Kent C Faul,
Kevin A Gonzalez,
Kham Phommachanh,
Kristin M Abaonza,
Lacresha D Chatman,
Ladislav Kermet,
Larry K Hampton,
Laura Fontan, MS,
Laura L Staples,
Lauren L Vajo,
Laurie B Frazier,
Lawrence J Stringer,
LCDR Debra Emerson,
LCDR Michael H Tollon,
LCDR Randall L Morris,
Lesley K Satterwhite,
Leslie A Cartmill,
Libia M Lugo,
Lillian Mcolon Mcknight,
Linda K Matheny,
Lindsay R Hatch,
Lindsay R Mundy,
Lindsey M Schwierjohann,
Lisa A Warner,
Lisa R Hilliar,
Lissette Paiz,
Lizaida E Rodriguez,
Lourdes Andujar,
LT Daveta L Bailey,
LT Kimberly M Hull,
LT Richard A Lyght,
LT Tamara J Henderson,
Lucas B Leake,
Luis A Dasta,
Luis Mburgos Medero,
Lundy H Patrick,
Lura D Baquero,
Lynne Ensor,
M Edith Snyder,
Marc Balzarini,
Marcellinus D Dordunoo,
Marcus A Ray,
Margaret M Annes,
Maria V Price,
Marie A Fadden,
Marie F Morin,
Marinee Flores Marrero,
Mark C Saale,
Mark E Parmon,
Marlene G Swider,
Mary A Millner,
Mary F Bodick,
Mary Jeanet Mcgarry,
Matthew B Casale,
Matthew B Thomaston,
Megan T Ziegler,
Meisha R Sampson,
Meisha Waters,
Melanie G Warzala,
Melanie M Walker,
Melanie W Pishnery,
Melba Trivera Clavell,
Melinda B Lewis,
Melissa D Ray,
Melissa J Garcia,
Melissa J Hill,
Mendoza,
Meredith M Andress,
Meredith M Cobb,
Meyer J Slobotsky,
Michael A Charles,
Michael C Lombardi,
Michael Shanks, MS,
Michael T Cyrus,
Michele L Obert,
Michele Perry Williams,
Michelle K Cockrell,
Miguel A Martinez Perez,
Mihaly S Ligmond,
Mindy M Chou,
Mizanne E Lewis,
Monica Cburgos Garcia,
Moraima Jramos Valle,
Mra Davism,
Mra M Barbosar,
Mra Mcculloughj,
Munoz,
Muralidhara B Gavini, PhD,
Nakesha J Jackson,
Narong Chamkasem, PhD,
Naveen B Kanthamneni,
Nawab A Siddiqui,
Nebil A Oumer,
Nelson E Venerio,
Nicolas Riveratorres,
Nicole A Lloyd,
Nicole E Knowlton,
Noreen Muñiz,
Omotunde O Osunsanmi,
Ormond,
Pankaj H Amin,
Paraluman S Leonin,
Parul M Patel,
Patty P Kaewussdangkul,
Paul A Bonneau,
Paul L Figarole, Jr,
Penny H Mccarver,
Peter C Chow,
Philip F Istafanos, DMV, MS,
Philippa Hillyer,
Postelle Birch Smith,
Qin Xu,
Rachael L Cook,
Rachel C Harrington,
Rafael Nevarez Nieves,
Ramon A Hernandez,
Raquel Gonzalez Rivera,
Rebecca E Dombrowski,
Rebecca Rodriguez,
Regina T Brown,
Richard E Needham,
Richard K Vogel,
Richard Ledwidge (nmi), PhD,
Richmond K Yip,
Ricki A Chase,
Robert C Coleman,
Robert D Tollefsen,
Robert J Maffei,
Robert J Martin,
Robert W Calais,
Robin Levis, PhD,
Rochelle K Kimmel,
Ronald T Weber,
Rosario D'costa,
Roy R Rinc,
Rozelle G Smith,
Saleem A Akhtar,
Salvatore N Randazzo,
Samina S Khan,
Sandra Carpio,
Sarah E Mcmullen,
Sarah Ibrahim,
Saundrea A Munroe,
Sayeeda Hdabe,
Scott T Ballard,
Seneca D Toms,
Sharna D Pratt,
Sharon Giamberini,
Sharon K Thoma, PharmD,
Shawn R Gould,
Sherbet L Samuels,
Shirshendu K Deb, PhD,
Sidney B Priesmeyer,
Simone E Pitts,
Song Y Lavalais,
Sonia M Monges,
Sony Mathews,
Sonya M Edmonds,
Stacey F Allard,
Stacey F Rivette,
Stacie A Woods,
Stephanie A Slater, MS,
Stephanie C Milan,
Stephanie D Crockett,
Stephanie Mangigian, MS/OSH, RN,
Steven A Brettler,
Steven D Dittert,
Steven D Kehoe,
Steven J Thurber,
Steven P Donald,
Susan D Yuscius,
Susan F Laska, MS,
Susan M Corrales,
Susan M Jackson,
Susan M Turcovski,
Susan T Hadman,
Susan W Ting,
Suzanne N Vallez,
Swati Verma,
Tajah L Blackburn,
Tamala P Bogan,
Tamala P Magee,
Tamara M Casselman,
Temar Q Williams,
Teresa M Hillebrandt,
Thai D Truong,
Thomas J Arista,
Thomas R Withers,
Tina M Pawlowski,
Torrance J Slayton,
Tracy L Reed,
Truong Xuan Nguyen (Andy),
Unnee Ranjan,
Uttaniti Limchumroon (Tom),
Vernon L O'neil,
Victor Spanioli,
Vincent Thomas,
Virginia L Meeks,
Viviana Matta,
Vivin George,
Vlada Matusovsky,
Walden H Lee,
Wanda A Lenger,
Warren J Lopicka,
Wayne D Mcgrath,
Wen Ning Chan (Sally),
Xiaokuang Lai, PhD,
Yasamin Ameri,
Yifan Wang,
Youmin Wang,
Yumi J Hiramine,
Zachary A Bogorad,
Zhaoyang Meng,
Zhong Li, PhD,
Zhongren Wu
Jessica L Pressley's Documents
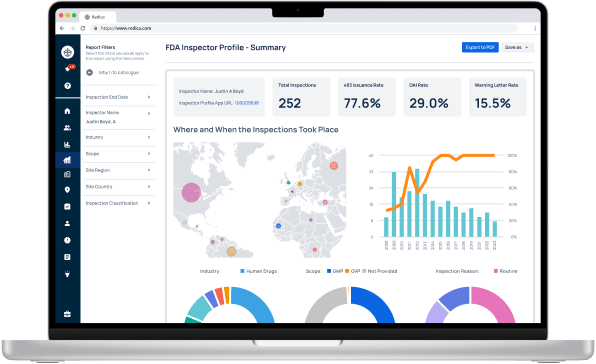
Experience Redica Systems’ NEW investigator profiles and dashboards
Redica Systems customers get access to over 1,400 FDA Investigator profiles, containing powerful analysis like:
- Total inspections conducted
- 483 rate
- OAI rate
- Warning Letter rate
- Inspections by Industry, Scope, and Inspection Reason
- And much more
Investigator Profiles are not sold as on-demand documents. If you’d like to see one, fill out the form below to connect with our Sales Team and get it in your inbox as little as 24 hours.
Talk to sales to access our investigator profiles
Loading...
Investigator Profiles Are Just the Tip of the Iceberg
Becoming a Redica Systems customer not only provides you with unlimited access to profiles similar to the one above for every current and former FDA Investigator, but also grants you access to:
Inspection Intelligence
- Pre-Approval Inspection (PAI) trends
- The ability to filter nearly any FDA enforcement action by GxP labels like GMP, GCP, etc.
- Latest 483s: see what the trends are across all FDA Investigators
- CFR Heatmap: Citations aggregated by Subpart by Year
Vendor Intelligence
- Full inspection histories for your vendors down to the specific site level
- All of the functionality mentioned above but isolated to just your vendors
Regulatory Intelligence
- Zero in on the trends for a specific agency/regulator (ex. FDA, MHRA, EMA, Health Canada)
- All the latest "Signals" from agencies around the world, segmented by Country, Type (ex. Guidance, Decree...), Category (ex. Rules/Regulations/Guidance, News, Reports...), Theme (ex. Cell and Gene Therapy, AI...), and more