FDA Investigator Raymond T Oji
Raymond T Oji has conducted inspections on 74 sites in 11 countries as of 04 May 2006. See below for a list of the FDA enforcement documents resulting from those inspections.
Investigator Details
Number of Inspected Sites:
74
Last Inspection Date:
04 May 2006
Investigator Role:
FDA Investigator
Redica ID:
Countries of Inspections:
United States of America,
France,
Switzerland,
Sweden,
India,
Belgium,
Germany,
Japan,
Italy,
Israel,
Canada
FDA Investigators that have inspected at least one site in common with Raymond T Oji:
Adam R Cooke,
Alan P Kurtzberg,
Alberto A Viciedo,
Alice S Tsao,
Alicia K Mckinsey,
Alicia M Mozzachio,
Alla Dubrovsky,
Almaris N Alonso,
Amanda L Fyles,
Amir Alavi,
Andrace L Deyampert,
Andrea A Branche,
Andrea D Swingle,
Andrea George, PhD,
Andrew P Lin,
Andrew S Limson,
Andrew W Harmon,
Angel K Sandhu,
Anh M Lac,
Ann L Demarco,
Anna Lazar,
Annemarie Bodnar,
Anthony F Lorenzo,
Ariel Cruz Figueroa,
Ashar P Parikh,
Atul J Agrawal,
Azza Talaat,
Barbara J Wilcox, PhD,
Barbara Janine Breithaupt,
Barbara M Frazier,
Bei Y He,
Benjamin J Smith,
Bichsa T Tran,
Binh T Nguyen,
Bonita S Chester,
Bonnie E Conley,
Bonnie E Pierson,
Brett R Havranek,
Bryan A Galvez,
Burnell M Henry,
Byungja E Marciante,
Calvin B Koerner,
Candace Y Gomez Broughton,
Carl A Anderson,
Carl A Huffman, III,
Carl Lee,
Carla A Norris,
Carla J Lundi,
Carole L Jones,
Carolyn A Renshaw,
Caryn M Everly,
Caryn M Mcnab,
Catherine J Laufmann,
CDR Ileana Barreto Pettit,
CDR Rochelle B Young, RPh, MSA,
Chaoying C Ma,
Charles I Ann,
Charles L Larson,
Charles L Zhou,
Charles M Edwards,
Charles R Cote, RIC,
Chelsea N Sealey,
Cheryl A Clausen,
Chris A Sack,
Christine M Parmentier,
Christopher R Czajka,
Christopher T Peters,
Claudia Mperez Kasmarski,
Comyar Shoghi,
Constantin Y Philopoulos,
Corrine M Carter,
Dana M Klimavicz,
Dandan Wang, PhD,
Daniel C Geffin,
Daniel J Lahar,
Daniel J Roberts,
Daniel R Solis,
Darlene B Almogela,
David A Paterson,
David K Lau,
David M Beltran,
David Serrano,
Debara R Reese,
Debara Reese,
Deborah M Trout,
Demitria J Xiradakis,
Denise L Burosh,
Denise M Digiulio,
Dennis Cantellops Paite,
Dennis E Guilfoyle, PhD,
Diana M Rand,
Diane L Raccasi,
Dina A Tallman,
Dipesh K Shah,
Donald C Obenhuber, PhD,
Donna Mwilliams Hill, PhD,
Douglas A Campbell,
Dr. Barbara D Paul, PhD,
Dr. Carmelo Rosa,
Dr. Jason R Caballero,
Dr. Mark J Seaton, PhD,
Dr. Ralph M Bernstein, PhD,
Dr. Sriram Subramaniam, PhD,
Dr. Zhihao Qiu (Peter), PhD,
Dr. Zhou Chen (nmi), MD PhD,
Dustin P Tran,
Dustin R Abaonza,
Dyana K Stone,
Edmund F Mrak, Jr,
Edwin Martinez,
Eileen A Liu,
Elizabeth M Jacobson,
Emal Wahab,
Emest F Bizjak,
Emily J Orban,
Emily Shacter,
Emmanuel Adu Gyamfi, PhD,
Eric L Dong, BS,
Eric M Mueller, PharmD,
Eric S Weilage,
Eric W Anderson,
Erin C Hill,
Evelyn Taha,
Evelyn Wong,
Farhana Khan,
Felix Maldonado,
Frederick F Razzaghi,
Frederick L Fricke,
Gavin T Cua,
Gene R Gunn,
Gerald Feldman,
Gerald M Feldman, PhD,
Gerald N Mcgirl, DDS,
Gina M Hall,
Gregory A Berg,
Gregory Price,
Gregson A Joseph,
Gwyn G Dickinson,
Henry K Lau,
Hung H Do, MS,
Iraida Ortiz,
Israel Santiago, OCM,
Italia Nappi,
Jacob G Lutz,
James C Lee, PharmD,
James L Dunnie, Jr,
James R Evans,
Jamie P Webb,
Jane S Wernberg,
Janet D Blount,
Jannifer L Degroot,
Javier O Vega,
Jawaid Hamid,
Jean Blackston Hill,
Jeanne Fringer, PhD,
Jeffery A Johnson,
Jeffrey A Sommers,
Jeffrey D Meng,
Jeffrey M Watson,
Jeffrey P Raimondi,
Jeffrey W Shrifter, DPM,
Jennifer A Kemp,
Jennifer L Johnson,
Jennifer Lalama,
Jennifer M Gogley,
Jinnie Kokiatkulkij,
Joan T Briones,
Joanne Hosting,
Jocelyn E Massey,
Jocelyn E Sparks,
Joe X Phillips,
Joey V Quitania,
Jogy George,
John A Gonzalez,
Jolanna A Norton,
Jose A Lopez,
Jose Acruz Gonzalez,
Jose R Hernandez,
Joseph A Piechocki,
Joseph Kutze, PhD,
Joseph P Iser, MD,
Joseph R Strelnik,
Joseph X Phillips,
Joshua S Hunt,
Joslyn K Brunelle, PhD,
Juanita P Versace,
Justin A Boyd,
Karen D Phung,
Karen E D'orazio,
Karen M Montgomery (KMM),
Kari M Johansen,
Katherine E Jacobitz,
Katherine Szestypalow,
Katie L Penno,
Kelvin Cheung,
Kenneth M Gordon,
Kent C Faul,
Kevin A Gonzalez,
Kevin P Foley,
Kham Phommachanh,
Khoa Nathanv Tran,
Kim Lthomas Cruse,
Kimberly L Anderson,
Kristen D Evans,
Kristin M Abaonza,
Kula N Jha,
L'oreal F Walker,
Lakisha M Williams,
Lance Mde Souza, MBA,
Larry K Austin,
Lata C Mathew, PhD,
Laura Fontan, MS,
Lauren Iacono Connors, PhD,
Laurie A Haxel,
Laurie P Norwood,
Laurimer Kuilan Torres,
Lawrence J Stringer,
Lawton W Lum,
LCDR Chad N Thompson,
LCDR Debra Emerson,
LCDR Jennifer H Rhyu,
Leigh Anne Myers,
Lequita M Mayhew,
Leslie D Wagner,
Lija V Fellows,
Linda F Murphy,
Linda Linh N Adams,
Linda Thai,
Linh Tu,
Lisa M Bellows,
Lisa Shin,
Lori J Silverstein,
Lorna F Jones,
Louis B Cencetti,
Lucila B Nwatu,
Luella J Rossi,
Luis A Dasta,
Lydia I Rosas Marty,
Lynda L Perry, PhD,
Maan S Abduldayem,
Mabel M Lee,
Maida Henesian (NMI),
Makini Cobourne Duval, PhD,
Marco S Esteves,
Marcus F Yambot,
Margaret M Annes,
Marie F Morin,
Marie Falcone,
Marie K Kinkade,
Marie T Falcone,
Mariza M Jafary,
Mark A Tucker,
Mark C Saale,
Mark E Chan,
Mark R Mcclain,
Marsha W Major,
Marshalette O Edwards,
Martin K Yau, PhD,
Mary E Storch,
Mary R Hole,
Matthew J Johnson,
Matthew J Sienko,
Matthew R Clabeaux,
Megan A Haggerty,
Meisha R Sampson,
Meisha Waters,
Melina Lrodriguez Upton,
Melissa J Garcia,
Mercy Oyugi,
Meyer J Slobotsky,
Michael A Charles,
Michael A Taylor,
Michael D Garcia,
Michael R Goga,
Michael R Klapal,
Michael S Araneta,
Michael Shanks, MS,
Michael T Cyrus,
Michele L Forster, PhD,
Michele L Obert,
Michele Perry Williams,
Michelle Rfrazier Jessen, PhD,
Mihaly S Ligmond,
Min Shanmabel Liu,
Mohsen Rajabi Abhari, FDA,
Montgomery Montgomery, Karen M,
Mra B Kamberem,
Mra Davism,
Mra Mcculloughj,
Mra Munizn,
Muna Algharibeh,
Muralidhara B Gavini, PhD,
Myra K Casey,
Nailing Zhang,
Nancy G Schmidt,
Navneet Kaur,
Nayan J Patel,
Neali H Lucas,
Nicholas L Hunt,
Nicholas L Paulin,
Nicholas Obiri, PhD,
Nicole A Lloyd,
Nicole E Knowlton,
Niketa Patel,
Nilufer M Tampal, PhD,
Noreen Muñiz,
Omotunde O Osunsanmi,
Otto D Vitillo,
Pal S Mayasandra,
Paraluman S Leonin,
Parul M Patel,
Patricia A Cochran,
Patricia H Dlugosz,
Patrick B Cummings,
Patrick C Klotzbuecher,
Patsy J Domingo,
Paul E Stein,
Paul L Bellamy,
Paul L Figarole, Jr,
Paul Mouris,
Paula A Trost,
Paula J Bretz,
Perry H Gambrell,
Peter E Baker,
Peter Kessler, PhD,
Peter R Lenahan,
Philip F Istafanos, DMV, MS,
Prabhu P Raju,
Qiao Y Bobo,
Qin Xu,
Quynh H Strandberg,
Rachel C Harrington,
Rafael E Arroyo,
Rafeeq A Habeeb,
Ralph A Erickson,
Randall N Johnson,
Ravi S Harapanhalli,
Reba A Gates,
Rebecca E Dombrowski,
Rebecca Rodriguez,
Rebecca T Davis,
Regina T Brown,
Richard E Needham,
Richard L Friedman,
Riley C Myers, PhD,
Robert C Coleman,
Robert C Steyert,
Robert D Tollefsen,
Robert J Doyle,
Robert J Ham,
Robert M Barbosa,
Rochelle K Kimmel,
Roger F Zabinski,
Rose Ashley,
Roy R Rinc,
Ruben C Ayala, PharmD,
Rumany C Penn, PharmD,
Russell J Glapion,
Russell K Riley,
Saied A Asbagh,
Saleem A Akhtar,
Sam H Haidar, PhD,
Samina S Khan,
Samuel T Walker,
Sandra A Boyd,
Sandra A Hughes,
Sandra S Saniga,
Sangeeta M Khurana, PhD,
Santiago Gallardo Johnson,
Sarah E Mcmullen,
Sarah E Rhoades,
Sarah Forney,
Satheesh Thomas,
Scott N Lim,
Sean R Marcsisin,
Seema S Singh,
Serge L Beaucage,
Sergio Chavez (NMI),
Seyyida Saterfield,
Shannon B Ruelle,
Sharon K Thoma, PharmD,
Sheilyn H Huang,
Shelley H Beausoleil,
Sherri J Jackson,
Shirley H Isbill,
Shuang Tang,
Sidney B Priesmeyer,
Simone E Pitts,
Sina Shojaee,
Sixto M Mercado Rios,
Sneha S Patel,
Sonya L Karsik, RAC,
Sparky L Bartee,
Stephanie A Slater, MS,
Stephen D Brown,
Stephen R Souza,
Steven C Madzo,
Steven D Kehoe,
Steven M Weinman,
Stuart W Russell,
Sudipan Karmakar,
Sue Lee Chan,
Susan D Yuscius,
Susan F Laska, MS,
Susan M Jackson,
Susan P Bruederle,
Suyang Qin,
Taichun Qin, PhD,
Tamala P Magee,
Ted L Anderson,
Temar Q Williams,
Terri L Dodds,
Thea C Grome,
Thomas J Arista,
Thomas R Withers,
Thomas W Nojek,
Thuy T Nguyen, LCDR,
Timothy C Grome,
Timothy T Kapsala,
Tina M Pawlowski,
Tod J Merkel,
Todd M Bozicevich,
Truong Xuan Nguyen (Andy),
Uduak M Inokon,
Uttaniti Limchumroon (Tom),
Vilmary Negron Rodriguez,
Vioela J Caze,
Virgilio F Pacio, CSO,
Viviana Matta,
Viviana R Ramirez,
Walden H Lee,
Wayne E Seifert,
William D Tingley,
William J Leonard,
William V Millar,
Xiaoping Guan,
Yangmin Ning,
Yi Wang, PhD,
Ying Xin Fan, PhD,
Yiyue Zhang (nmi), PhD,
Yumi J Hiramine,
Yvette Mlacour Davis,
Yvonne E Lozano,
Zachary A Bogorad,
Zachary L Stamm,
Zhong Li, PhD
Raymond T Oji's Documents
Experience Redica Systems’ NEW investigator profiles and dashboards
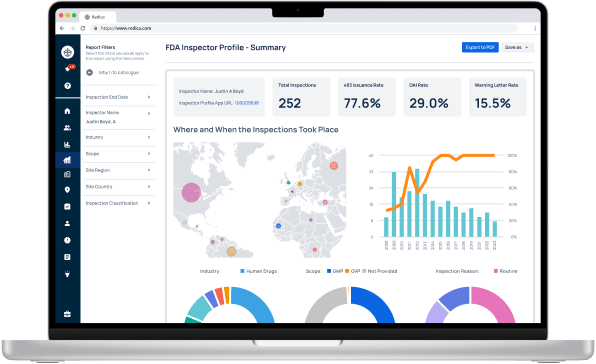
Redica Systems customers get access to over 1,400 FDA Investigator profiles, containing powerful analysis like:
- Total inspections conducted
- 483 rate
- OAI rate
- Warning Letter rate
- Inspections by Industry, Scope, and Inspection Reason
- And much more
Investigator Profiles are not sold as on-demand documents. If you’d like to see one, fill out the form below to connect with our Sales Team and get it in your inbox as little as 24 hours.
Talk to sales to access our investigator profiles
Loading...
Investigator Profiles Are Just the Tip of the Iceberg
Becoming a Redica Systems customer not only provides you with unlimited access to profiles similar to the one above for every current and former FDA Investigator, but also grants you access to:
Inspection Intelligence
- Pre-Approval Inspection (PAI) trends
- The ability to filter nearly any FDA enforcement action by GxP labels like GMP, GCP, etc.
- Latest 483s: see what the trends are across all FDA Investigators
- CFR Heatmap: Citations aggregated by Subpart by Year
Vendor Intelligence
- Full inspection histories for your vendors down to the specific site level
- All of the functionality mentioned above but isolated to just your vendors
Regulatory Intelligence
- Zero in on the trends for a specific agency/regulator (ex. FDA, MHRA, EMA, Health Canada)
- All the latest "Signals" from agencies around the world, segmented by Country, Type (ex. Guidance, Decree...), Category (ex. Rules/Regulations/Guidance, News, Reports...), Theme (ex. Cell and Gene Therapy, AI...), and more