FDA Investigator Wayne E Seifert
Wayne E Seifert has conducted inspections on 63 sites in 16 countries as of 30 May 2024. See below for a list of the FDA enforcement documents resulting from those inspections.
Investigator Details
Number of Inspected Sites:
63
Last Inspection Date:
30 May 2024
Investigator Role:
FDA Investigator
Redica ID:
Countries of Inspections:
Ireland,
United States of America,
United Kingdom of Great Britain and Northern Ireland,
Malaysia,
Korea (Republic of),
Italy,
Belgium,
France,
Germany,
Switzerland,
Spain,
Austria,
Denmark,
India,
Japan,
Netherlands
FDA Investigators that have inspected at least one site in common with Wayne E Seifert:
Aaron L Dunbar,
Adam R Cooke,
Ademola O Daramola,
Aditi S Thakur,
Aimee Cunningham, PhD,
Alan L Truong,
Alan R Condon,
Albert A Salvador,
Alberto A Viciedo,
Alice S Tsao,
Alicia M Mozzachio,
Amber D Brodas,
Amy C Jordan,
Amy Devlin, PhD,
Amy N Chen,
Anastasia G Lolas,
Anastasia M Shields,
Anders W Evenson,
Andrace L Deyampert,
Andrea A Branche,
Andrea D Swingle,
Andrea Siegel, PhD,
Andrew "Drew" Haack, PhD,
Andrew J Barrowcliff,
Andrew K Haack, PhD,
Andrew M Barlow,
Anh M Lac,
Anissa M Cheung,
Anita Narula, PhD,
Anjali Shukla, PhD,
Ann L Demarco,
Ann Marie Montemurro,
Antonina Aydanian,
Arie C Menachem,
Arsen Karapetyan,
Arulvathani P Arudchandran,
Ashar P Parikh,
Ashley N Queen, PhD,
Azza Talaat,
Baolin Zhang, PhD,
Barbara Janine Breithaupt,
Bei Y He,
Ben Du,
Benjamin J Smith,
Bijoy Panicker,
Bo Chi, PhD,
Bonita S Chester,
Brandon C Heitmeier,
Brandy N Lepage,
Brenda P King,
Brenda W Uratani, PhD,
Brentley S Collins,
Brian D Nicholson,
Brian M Janelsins, PhD,
Brittani N Franklin,
Brooke K Higgins,
Bruce H Mccullough,
Burnell M Henry,
Byungja E Marciante,
Calvin B Koerner,
Camerson E Moore,
Candace Y Gomez Broughton,
Carl A Huffman, III,
Carla A Norris,
Carla J Lundi,
Carolyn A Renshaw,
Carrie Ann Plucinski,
Cary Greene,
Caryn M Mcnab,
Catherine J Laufmann,
CDR Donald Ertel,
CDR Rochelle B Young, RPh, MSA,
CDR Thomas R Berry, PPh,
Charisse K Green,
Charles I Ann,
Charles Jewell,
Charles L Zhou,
Charles Yuanchia Kuo, PhD,
Chava Kimchi Sarfaty, PhD,
Chen Sun, PhD,
Chiang Syin, PhD,
Chikako Torigoe, PhD,
Christian D Lynch (CDL),
Christina A Miller,
Christina Capacci Daniel, PhD,
Christina K Theodorou,
Christina Theodorou,
Christine W Twohy,
Christopher D Leach,
Christopher Downey, PhD,
Christopher R Czajka,
Colleen F Hoyt,
Concepcion Cruz, Jr,
Constantin Y Philopoulos,
Corrine M Carter,
Craig A Garmendia,
Craig D Zagata,
Cristina Ausin Moreno, PhD,
Cynthia J Lee, MS,
Cynthia Jim, CSO,
Cynthia M Goudeau,
Cynthia White,
Daniel B Arrecis,
Daniel J Lahar,
Daniel L Zheng,
Daniel T Lee,
Dariusz Galezowski,
Daryl A Dewoskin,
David A Paterson,
David G Eng,
David M Beltran,
Dawn L Wydner,
Dawn M Braswell,
Debara R Reese,
Debara Reese,
Deborah M Trout,
Deborah Schmiel, PhD,
Debra L Pagano,
Demitria J Argiropoulos,
Demitria J Xiradakis,
Denise L Burosh,
Dennis Cantellops Paite,
Dennis E Guilfoyle, PhD,
Devaughn Edwards,
Diane L Raccasi,
Dianne S Alexander,
Dien N Nguyen,
Dina A Tallman,
Djamila Harouaka,
Doan T Nguyen, PharmD,
Dogbeda F Mackenzie,
Dolores Harper,
Don H Bark, PhD,
Donald C Obenhuber, PhD,
Donna A Katz,
Dorothy W Lee,
Douglas A Campbell,
Douglas C Kovacs,
Dr. Jun Liu, PhD,
Dr. Ralph M Bernstein, PhD,
Dr. Robert C Horan, MD,
Dr. Willie Wilson, PhD,
Dr. Zhihao Qiu (Peter), PhD,
Eboni S Funderburk,
Edmund F Mrak, Jr,
Edwin Jao,
Edwin Melendez,
Eileen A Liu,
Ekaterina Allen,
Eliezar Ramos,
Ellen P Madigan,
Emest F Bizjak,
Emilie E Kahn,
Emily J Orban,
Emine Guvenmaiorov,
Ephrem Hunde, PhD,
Erika M Wilkerson,
Erika V Butler,
Erin D Mccaffery,
Erin L Mcfiren,
Esther C Broner, PhD,
Felix Maldonado,
Francis A Guidry,
Frank Wackes,
Freyja V Lynn,
Gayle S Lawson,
Gene D Arcy,
George Pyramides,
George T Allen, Jr,
Gerald B Seaborn, Jr,
Gilbert T Salud,
Gloria E Parra,
Guerlain Ulysse,
Gunther Boekhoudt, PhD,
Gwyn G Dickinson,
Hai Lient Phung,
Hala L Selby,
Haley H Seymour,
Hamet M Toure, PharmD MPH,
Hao Kiet D Phan,
Haoheng Yan, PhD,
Helen B Ricalde,
Holly Brevig,
Howard Anderson, PhD,
Hyung Yul Lee,
Ibad U Khan,
J Paul Kirwan, PhD,
Jacek Cieslak, PhD,
Jacob G Lutz,
Jacqueline Mdiaz Albertini,
Jacqueline S Warner,
James A Lane,
James C Maclaughlin,
James D Bridges,
James I Giefer,
James L Dunnie, Jr,
James R Evans,
James W Whitney,
Jason F Chancey,
Javier E Santos,
Jawaid Hamid,
Jay T Wong,
Jean Blackston Hill,
Jee Chung, PhD,
Jeffrey A Sommers,
Jeffrey D Meng,
Jeffrey P Raimondi,
Jennifer A Kemp,
Jennifer C Adams,
Jennifer C Johnson,
Jennifer L Bridgewater,
Jennifer M Gogley,
Jennifer Swisher, PhD,
Jens Fricke,
Jeremy W Rotton,
Jessica Chery,
Jessica Hankins, PhD,
Jianming Li,
Jie He,
Jing Fang,
Joan A Loreng,
Joan Johnson,
Joey V Quitania,
John D White,
John P Mistler,
John S Hartford,
Jon P Antoniou,
Jonathan G Matrisciano,
Jonathan G Swoboda,
Jonathan W Chapman,
Jose A Lopez,
Jose Acruz Gonzalez,
José E Meléndez,
Jose M Melendez,
Jose Ohernandez Guzman,
Jose R Hernandez,
Joseph A Piechocki,
Joseph F Owens,
Joseph Kutze, PhD,
Joseph R Strelnik,
Joslyn K Brunelle, PhD,
Joyce A Rockwell,
Julianne C Mccullough,
Julie D Bringger,
Junho Pak,
Justin A Boyd,
Justine M Corson,
Kalavati Suvarna, PhD,
Kara A Scheibner, PhD,
Karen A Briggs,
Karen L Kosar,
Karen M Montgomery (KMM),
Karishma G Gopaul,
Katherine D Adams,
Katherine E Jacobitz,
Katherine Szestypalow,
Kathleen M Jordan,
Kathleen R Jones, PhD,
Kathryn E King,
Kathy E Craven,
Kathy E Noe,
Kent C Faul,
Kevin A Gonzalez,
Kevin D Kallander,
Kevin P Foley,
Kham Phommachanh,
Kim Lthomas Cruse,
Kirsten L Vadheim, PhD,
Ko U Min,
Kristin M Abaonza,
Kristina L Conroy,
Kshitij A Patkar,
Kula N Jha,
Kumar G Janoria,
Kurt A Brorson, PhD,
L Norwood,
L'oreal F Walker,
Lakshmi Narasimhan, PhD,
Lane V Christensen, PhD,
Larry K Austin,
Lata C Mathew, PhD,
Latoya A Griffith,
Laura Fontan, MS,
Laura I Salazar, PhD,
Laurel A Beer,
Lauren Iacono Connors, PhD,
Laurie Graham,
Laurimer Kuilan Torres,
Lawrence J Stringer,
LCDR Chad N Thompson,
LCDR Debra Emerson,
LCDR Mark A Jimenez, RN,
LCDR Sean Byrd,
Lei Zhang, PhD,
Leigh Anne Myers,
Leiyun Boone, PhD,
Leon L Law,
Lewis K Antwi,
Li Lu, PhD,
Linda F Murphy,
Linda M Cheny,
Linda Thai,
Lindsey Brown, PhD,
Lisa B Orr,
Lisa K Capron,
Lisa M Bellows,
Lixia Cai,
Logan T Williams,
Lois K Gross,
Lori A Holmquist,
Lori Peters,
Lorie S Hannappel,
LTJG Bradley E Benasutti,
Lucila B Nwatu,
Luella J Rossi,
Luis Mburgos Medero,
Lydia S Chan,
Lynda L Perry, PhD,
M Pedgen,
Maan S Abduldayem,
Madushini Dharmasena, PhD,
Maida Henesian (NMI),
Maotang Zhou, PhD,
Marcellinus D Dordunoo,
Marcelo O Mangalindan, Jr,
Marcus A Ray,
Marcus F Yambot,
Margaret E Sarles,
Margaret M Annes,
Margarita Santiago,
Maria Cecilia Tami, PhD,
Maria Cruz Fisher,
Maria Gutierrez Hoffman, PhD,
Maria Gutierrez Lugo, PhD,
Maria Joselopez Barragan, PhD,
Marie B Buen Bigornia,
Marie F Morin,
Marion Michaelis,
Marisa E Stock,
Mariza M Jafary,
Marjorie A Shapiro, PhD,
Mark Brunswick, PhD,
Mark C Saale,
Mark R Mcclain,
Marlene G Swider,
Marsha W Major,
Martha Sullivan Myrick,
Martha T Olone,
Martin R Vowell,
Mary Davis Lopez,
Mary E Farbman, PhD,
Mary E Storch,
Mary Jeanet Mcgarry,
Maryam Tabatabaie,
Massoud Motamed,
Mate Tolnay,
Matthew A Spataro,
Matthew B Casale,
Maxwell Van Tassell, PhD,
Maya M Davis,
Megan A Haggerty,
Mei Chiung Huang (Jo),
Meisha R Sampson,
Meisha Waters,
Melanie W Pishnery,
Melina Lrodriguez Upton,
Melissa D Ray,
Mercy Oyugi,
Michael A Charles,
Michael D Omeara,
Michael E Maselli,
Michael L Chasey,
Michael P Anthony,
Michael R Goga,
Michael R Klapal,
Michael S Araneta,
Michael Shanks, MS,
Michele L Forster, PhD,
Michele L Glendenning,
Michele L Obert,
Michele Perry Williams,
Michelle M Noe Varga,
Michelle Yclark Stuart,
Mihaly S Ligmond,
Mikhail V Ovanesov, PhD,
Milan S Blake,
Milos Dokmanovic, PhD,
Milva E Melendez Perales,
Mindy M Chou,
Mizanne E Lewis,
Monica Commerford, PhD,
Monica Markovski, PhD,
Monique C Lo,
Montgomery Montgomery, Karen M,
Mra B Kamberem,
Mra Davism,
Mra Mcculloughj,
Mra Munizn,
Mra V Millarw,
Muna Algharibeh,
Murthy Vkn Sikha,
Myra K Casey,
Nailing Zhang,
Nancy A Bellamy,
Nancy L Rolli,
Nancy T Waites,
Nataniel Phillips Sylvain, PhD,
Neali H Lucas,
Nealie C Newberger,
Nebil A Oumer,
Nicholas F Lyons,
Nicholas L Paulin,
Nicole A Lloyd,
Nicole K Trudel,
Niketa Patel,
Noreen Muñiz,
Obinna R Echeozo,
Ogechi C Nna,
Olumide A Martins,
Omotunde O Osunsanmi,
Pablo Alcantara,
Pankaj H Amin,
Paraluman S Leonin,
Parul M Patel,
Patricia A Cochran,
Patricia D Stahnke,
Patricia F Hughes, PhD,
Patricia H Dlugosz,
Patrick B Cummings,
Patrick C Klotzbuecher,
Patrick J Lynch,
Patrick L Wisor,
Patsy J Domingo,
Patty P Kaewussdangkul,
Paul A Bonneau,
Paul L Bellamy,
Paul L Figarole, Jr,
Paul M Kawamoto,
Paul Mouris,
Paula A Trost,
Peng Zhou,
Perry T Nichols,
Pete Amin (Pankaj),
Peter Adams, PhD,
Peter R Lenahan,
Philip F Istafanos, DMV, MS,
Phillip Angart, PhD,
Prabhu P Raju,
Prajakta A Varadkar,
Priscilla M Pastrana,
Qiao Y Bobo,
Qin Xu,
Qing Joanna Zhou, PhD,
Rabia Ballica, PhD,
Rachael O Oyewole,
Rafael Padilla,
Rafeeq A Habeeb,
Rajiv R Srivastava,
Ralph A Erickson,
Ralph H Vocque,
Ram Sihag, PhD,
Ramon E Martinez,
Randy L Self,
Ranjani Prabhakara,
Raquel Gonzalez Rivera,
Rashmi Rawat, PhD,
Raymond T Oji,
Rebecca E Dombrowski,
Rebecca Rodriguez,
Regina T Brown,
Reginald D Neal,
Reyes Candau Chacon, PhD,
Richard H Penta,
Richard Ledwidge (nmi), PhD,
Richard W Berning,
Richard W Thornton,
Riley C Myers, PhD,
Robert Barbosa,
Robert C Coleman,
Robert C Steyert,
Robert D Tollefsen,
Robert J Ham,
Robert J Maffei,
Robert J Martin,
Robert Jennings,
Robert L Stevenson,
Robert M Barbosa,
Roger F Zabinski,
Rong Guo,
Rory K Geyer,
Rose Ashley,
Roshni J Patel,
Roy R Rinc,
Rumany C Penn, PharmD,
Russell J Glapion,
Russell K Riley,
Ruth Moore, PhD,
S Lori Brown, PhD MPH,
Saleem A Akhtar,
Samantha J Bradley,
Samina S Khan,
Samuel J Arsenault,
Samuel Mindaye,
Sandra A Boyd,
Sang Bong Lee,
Sangeeta M Khurana, PhD,
Santos E Camara,
Sarah Arden,
Sarah B Tanksley,
Sarah Forney,
Sarah Kennett, PhD,
Sarah Nwandiuko,
Sargum C Sood,
Satheesh Thomas,
Scott C Lute,
Scott E Norris,
Scott N Lim,
Scott R Nichols, PhD,
Scott T Ballard,
Sean R Marcsisin,
Sena G Dissmeyer,
Seneca D Toms,
Seth A Mailhot,
Shana L Williams,
Shannon A Gregory,
Sharon K Thoma, PharmD,
Shawn E Larson,
Shawn R Gould,
Shirley J Berryman,
Sidney B Priesmeyer,
Simone E Pitts,
Sneha S Patel,
Sony Mathews,
Stacey S Degarmo,
Stefanie L Heckman,
Stefanie L Kremer,
Stephanie A Slater, MS,
Stephen D Brown,
Stephen D Eich,
Stephen R Souza,
Steven A Gonzales,
Steven B Hertz,
Steven C Madzo,
Steven Fong, MS, PhD,
Steven Kozlowski, MD,
Steven L Carter,
Steven M Weinman,
Steven P Donald,
Stuart W Russell,
Susan F Laska, MS,
Susan M Jackson,
Susan P Bruederle,
Susan T Hadman,
Susan W Ciani,
Susanna E Ford,
Susanne M Richardson, MS RAC,
Suyang Qin,
Svetlana A Shestopal,
Tamara L Ely,
Tamara M Casselman,
Tamil Arasu, PhD,
Tania E Vizcaino,
Tara L Greene,
Tara R Gooen,
Tawny L Colling,
Teegan Dellibovi Ragheb, PhD,
Temar Q Williams,
Terri L Dodds,
Thai D Truong,
Thomas A Peter,
Thomas J Arista,
Thomas R Withers,
Thuy T Nguyen, LCDR,
Tiara Nbrown Crosen,
Timothy J Schmidt,
Tina M Pawlowski,
Tina S Roecklein,
Tonnie L Carter,
Torrance J Slayton,
Tracey L Harris,
Travis S Bradley,
Truong Xuan Nguyen (Andy),
Tzanko Stantchev,
Uduak M Inokon,
Unnee Ranjan,
V Teres Speer,
Vaishali J Patel,
Vanessa Y Gelsey, PhD,
Vanessa Y Jacobs,
Veronica Fuentes, MS,
Victor J Gangi,
Vidya B Pai,
Vijaya L Simhadri,
Vilmary Negron Rodriguez,
Virgilio F Pacio, CSO,
Virginia Carroll, PhD,
Viviana Matta,
Vlada Matusovsky,
Walter N Lange,
Wayne T Smith,
Wendy G Tan, PhD,
William D Tingley,
William Hallett, PhD,
Xianghong Jing (Emily), PhD,
Xiaokuang Lai, PhD,
Xiaoping Guan,
Xu Michael Di, PhD,
Yan Wang,
Yangmin Ning,
Yanming An, PhD,
Yasamin Ameri,
Yehualashet A Gessesse,
Yetao Jin, PhD,
Yideng Liang,
Ying Xin Fan, PhD,
Yiwei Li,
Yongmin Liu,
Youmin Wang,
Yuan Chia Kuo,
Yumi J Hiramine,
Yun Wu, PhD,
Yvesna C Blaise,
Yvins Dezan,
Yvonne E Lozano,
Zachary A Bogorad,
Zachary L Miller,
Zerita White,
Zhao Wang, PhD,
Zhong Li, PhD,
Zhongren Wu,
Ziyang Su, PhD,
Zonglin Hu
Wayne E Seifert's Documents
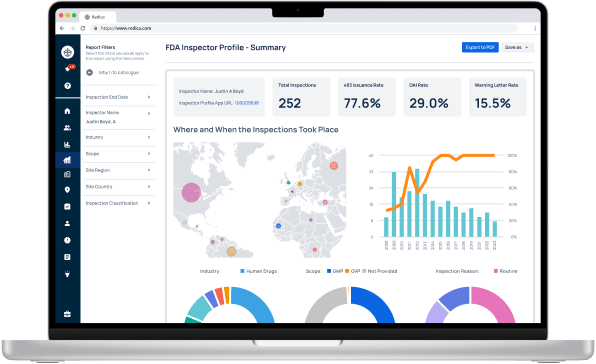
Experience Redica Systems’ NEW investigator profiles and dashboards
Redica Systems customers get access to over 1,400 FDA Investigator profiles, containing powerful analysis like:
- Total inspections conducted
- 483 rate
- OAI rate
- Warning Letter rate
- Inspections by Industry, Scope, and Inspection Reason
- And much more
Investigator Profiles are not sold as on-demand documents. If you’d like to see one, fill out the form below to connect with our Sales Team and get it in your inbox as little as 24 hours.
Talk to sales to access our investigator profiles
Loading...
Investigator Profiles Are Just the Tip of the Iceberg
Becoming a Redica Systems customer not only provides you with unlimited access to profiles similar to the one above for every current and former FDA Investigator, but also grants you access to:
Inspection Intelligence
- Pre-Approval Inspection (PAI) trends
- The ability to filter nearly any FDA enforcement action by GxP labels like GMP, GCP, etc.
- Latest 483s: see what the trends are across all FDA Investigators
- CFR Heatmap: Citations aggregated by Subpart by Year
Vendor Intelligence
- Full inspection histories for your vendors down to the specific site level
- All of the functionality mentioned above but isolated to just your vendors
Regulatory Intelligence
- Zero in on the trends for a specific agency/regulator (ex. FDA, MHRA, EMA, Health Canada)
- All the latest "Signals" from agencies around the world, segmented by Country, Type (ex. Guidance, Decree...), Category (ex. Rules/Regulations/Guidance, News, Reports...), Theme (ex. Cell and Gene Therapy, AI...), and more