FDA Investigator Iris C Macinnes
Iris C Macinnes has conducted inspections on 501 sites in 23 countries as of 03 Jun 2024. See below for a list of the FDA enforcement documents resulting from those inspections.
Investigator Details
Number of Inspected Sites:
501
Last Inspection Date:
03 Jun 2024
Investigator Role:
FDA Investigator
Redica ID:
Countries of Inspections:
United States of America,
United Kingdom of Great Britain and Northern Ireland,
Italy,
France,
Germany,
Canada,
Greece,
Finland,
Sweden,
Bulgaria,
Spain,
Russian Federation,
Denmark,
Belgium,
Croatia,
Ireland,
Japan,
Czechia,
Switzerland,
Portugal,
Poland,
Belize,
China
FDA Investigators that have inspected at least one site in common with Iris C Macinnes:
Abby Lmozeke Baker,
Adam C Hipko,
Akbar J Zaidi,
Akbar Javed,
Albert F Peacock, PhD,
Alcee M Tavarez,
Alexandra B Pitkin,
Allen F Hall,
Althea A Williams,
Amalia C Himaya,
Amanda J White,
Amanda K Willey,
Amanda L Fyles,
Amanda S Zorn,
Amir Alavi,
Andrea A Branche,
Andrew Saunders,
Angela Shepas,
Anh M Lac,
Anita Narula, PhD,
Annabelle Crusan, D,VM,
Anthony G Emerson,
Anya Dlockett Evans,
Arie C Menachem,
Arthur S Williams, Jr,
Ashar P Parikh,
Ashleigh P Barkans,
Ashleigh P Wodushek,
Ashley N Williams,
Audrey J Yarbrough,
Barbara Janine Breithaupt,
Barry Cherney, PhD,
Benjamin E Bowen,
Benjamin J Dastoli,
Betsy C Galliher,
Bichsa T Tran,
Bing Lin,
Binh T Nguyen,
Blondell W Johnson,
Bo Chi, PhD,
Bobbi J Miller,
Bonnie E Conley,
Bonnie E Pierson,
Brackens Harris,
Brandon O Neloms,
Brenda G Stewart Munoz,
Brian J Ryan,
Brien C Fox,
Byungja E Marciante,
Camerson E Moore,
Camille D Brown,
Carla J Lundi,
Carlos Chavez,
Carol S Sanchez,
Carolyn E Barney,
Carrie A Hughes,
Caryn M Mcnab,
Catherine J Laufmann,
CDR Sean T Creighton,
Charles B Steinmiller,
Charles D Brown,
Charles L Larson,
Cheron M Portee,
Christina C Santos,
Christina D Mello,
Christina V Santos,
Christine I Shaw,
Christine M Whitby, CSO,
Christopher D Rush,
Christopher J Hurst,
Christopher J Smith,
Claire M Minden,
Cody D Rickman,
Colleen E Burke,
Comyar Shoghi,
Connie L Hannon Rabel,
Crystal Monroy,
Crystal S Harris,
Cynthia A Harris, MD, RN,
Cynthia L Gorveatt,
Cynthia T Cain,
Dallas E Gilbreath Jr,
Dana M Klimavicz,
Daniel J Lahar,
Daniel S Fam,
Daniel W Cline,
Daniell Gill, Jr,
Danielle Lyke,
Dannie E Rowland,
Danny D Horner,
Darla J Christopher,
David Castillo,
David M Beltran,
David M Wilkinson,
David Serrano,
Davinna Ligons, PhD,
Dawn C Olenjack,
Denise Connelly,
Deniza Karacic,
Dennis R Butcher,
Diana K Krepel,
Diana L Ayala,
Diane Cvan Leeuwen,
Doan T Nguyen, PharmD,
Dolores Harper,
Don A Leeseberg,
Donna Ltartaglino Besone,
Dorothy W Lee,
Dr. Jason R Caballero,
Dr. Sriram Subramaniam, PhD,
Dylan R Jock,
Dyvette Arline,
Earl Echon,
Ed Gonzalez Vazquez,
Edmiston,
Edward D Edmiston,
Edward E Lockwood (EEL),
Edward H Maticka,
Elisa M Fleming,
Elizabeth Ad Girard,
Elizabeth B Griffin,
Elizabeth D Connell,
Ellen J Kleintop,
Ellen J Tave,
Elmina E Akwo,
Elmina E Mboh,
Enspect User,
Eric W Anderson,
Erica Uribe,
Ernest Serna, III,
Esther A Ofori,
Esther C Broner, PhD,
Everard A Irish,
Felix J Marrero,
Florecia S Philogene,
Frances Namuswe, PhD,
Francis A Guidry,
Francis J Eng,
Frederic W French, III,
Gene R Gunn,
George J Flynn,
German Rivera,
Gery M Duparc,
Gilbert Valdez, RS,
Gloria A Milster,
Gloria Jane Acosta,
Grant L Davis,
H Ljamilla Selby,
Habacuc V Barrera,
Hala L Selby,
Haley H Seymour,
Hamed Ghoda,
Haroon Vohra (NMI),
Hasan A Irier, PhD,
Himanshu Gupta, PhD,
Homero W Aguilar,
Hossein Khorshidi, PhD,
Hung V Le,
J Elkins,
Jacey Roy,
Jacqueline A O'shaughnessy, PhD,
Jacqueline S Warner,
James A Beaulieu,
James B Arnett,
James D Planchon,
James H Robinson,
James K Kane, PhD,
James M Odonnell,
James P Finn,
James R Fleckenstein,
James R Montero,
James W Blakely,
James W Whitney,
Jamie M Bumpas,
Jamie P Webb,
Janet B Abt,
Janet Pulver,
Janete F Guardia,
Janice M Hickok,
Janie Fuller,
Javelle P Spann,
Javier O Vega,
Jd Young,
Jeanne J Thai,
Jee Chung, PhD,
Jeffrey J Thibodeau,
Jeffrey P Raimondi,
Jeffrey R Wooley,
Jeffrey W Shrifter, DPM,
Jennifer D Young,
Jennifer Gogley,
Jennifer Hua,
Jennifer M Gogley,
Jennifer O Dowdy,
Jeremy L Stukes,
Jeremy W Rotton,
Jessica E Hensley,
Jinnie Kokiatkulkij,
Joan A Loreng,
Joanne M Schlossin,
Jocelyn C Turner,
Jocelyn E Massey,
Joel D Martinez,
Joel Welch, PhD,
Johann M Fitch,
John A Gonzalez,
John A Sciacchitano,
John L Stevens,
John R Myung,
Johnson,
Jonaida Estevez Martinez,
Jonathan A Womack,
Jose Acruz Gonzalez,
Jose Martinez, Jr,
Joseph A Seitz, III,
Joseph R Strelnik,
Joshua J Silvestri,
Joy L Ramsey,
Juan D Lopez,
Juanj N Wu,
Junho Pak,
K Gonzlez,
Ka L Wong,
Kamara Tx,
Karen A Coleman,
Karen C Daugherty,
Karen M Kondas,
Karlton T Watson,
Katelyn Astaub Zamperini,
Kathleen Chmura Shaffer,
Kathleen R Jones, PhD,
Katlin N Stubbs,
Kellia N Hicks,
Kelly D Moore,
Kelly D Sheppard,
Kelvin Cheung,
Kelvin X Sanders,
Kendra A Biddick,
Kenitra D Hewitt,
Kham Phommachanh,
Kim Lthomas Cruse,
Kimberly Lewandowski Walker,
Kirtida Patel,
L Grant,
Laiza V Garcia,
Lakecha N Lewis,
Lan T Tran,
Lance D Johnson,
Lance Sindo, CIH, CQA, RS,
Laura Fontan, MS,
Lauren E Priest,
Lauren E Skokan,
Lauren M Lilly, PhD,
LCDR Ismael Olvera, IV,
Leighton Kn Gai,
Leonard H Lavi,
Li Li,
Lillian S Wu,
Lillie M Young,
Lilly O Barton,
Linda Linh N Adams,
Lindsay R Hatch,
Ling Yu L Liu,
Lisa A Doyle,
Lisa M Puttonen,
Lisa P Oakes,
Lisa R Jennings,
Lloyd D Payne,
Lorie S Hannappel,
LT Melanie M Mayor, USPHS,
Lucas B Leake,
Luis E Gonzalez,
Maira P Brading,
Maotang Zhou, PhD,
Marc R Dickens,
Marcia B Williams,
Marcus F Yambot,
Margaret M Annes,
Maribeth G Niesen,
Marilyn S Babu,
Mark A Guerra,
Mark C Saale,
Mark W Babbitt,
Marla A Cassidy,
Marlo Ianm Alintanahin,
Martin K Yau, PhD,
Marvin D Jones,
Mary A Millner,
Maxyne T Lam,
Melissa G Gonzalez,
Merelynn Rhoten,
Miaja Umaedi,
Michael A Taylor,
Michael F Skelly, PhD,
Michael T Cyrus,
Michelle A Marsh,
Michelle J Hines,
Michelle Paxon,
Mihaly S Ligmond,
Mohsen Rajabi Abhari, FDA,
Monica Cburgos Garcia,
Monica J Wilkins,
Monique Rac Lester,
Msdap Gonzlezk,
Nathan R Moon,
Nicholas Obiri, PhD,
Nicholas Z Lu,
Nicola M Fenty Stewart,
Nilufer M Tampal, PhD,
Norman Wong,
Olalere D Fasipe,
Omotunde O Osunsanmi,
Patrice S Hall,
Patricia M Jacobs,
Patrick D Stone, MS,
Patty P Kaewussdangkul,
Paul E Frazier,
Paul M Kawamoto,
Paul W Moy,
Paula J Perry,
Pauline N Logan,
Pawan K Jain,
Perry H Gambrell,
Peter S Diak,
Philip F Istafanos, DMV, MS,
Philip M Steele, PhD,
Phillip D Waldron,
Phillip M Pontikos,
Pitman,
Pp Thorsten Feil,
Rachel C Stanton,
Ralph H Vocque,
Reba A Gates,
Rebeca Ulloa,
Rebecca Co Bryan,
Rebecca L Stephany,
Rebecca Rodriguez,
Reggie D Cope,
Rendall E Barfoot,
Rene R Ramirez,
Rhonda L Dash,
Rian L Pope,
Richard K Vogel,
Richard L Mallonee,
Richard L Rutherford,
Richard T Riggie,
Richmond K Yip,
Robert C Coleman,
Robert D Tollefsen,
Robert Darius,
Robert M Tillman,
Robert T Lorenz,
Rodney T Allnutt,
Roger F Zabinski,
Rona Leblanc, PhD,
Ronald L Koller,
Ronda Leblanc,
Ronda Rloyd Jones,
Ronnie Garza,
Rose Ashley,
Roy Baby,
Ruth E Medcalf,
Saijal P Naik,
Samantha J Bradley,
Samuel A Hamblin,
Samuel R Carter,
Sandra Jacquez,
Sandy J Ziegler,
Sara A Valenzuela,
Sara M Onyango,
Sarah A Hassas,
Sarah Arden,
Sarah J Cullen,
Sarah J Phillips,
Scott B Laufenberg,
Scott J Trsenjak,
Scott K Zika,
Scott R Nichols, PhD,
Scott T Ballard,
Sean M Cheney,
Selina M Mata,
Sha'tina R Alridge,
Shanna N Purdy,
Shaquenta Y Perkins,
Shawn E Larson,
Shelley H Beausoleil,
Sherrie L Krolczyk,
Sidney B Priesmeyer,
Sonia R Peterson,
Sonya L Karsik, RAC,
Srinivas R Chennamaneni, PhD,
Staci G Mcallister,
Stanley Au,
Stefan Preiss,
Stephanie T Durso,
Stephen C Smith,
Stephen D Brown,
Stephen D Eich,
Stephen L Beekman,
Steven D Dittert,
Steven Fong, MS, PhD,
Sue Lee Chan,
Sunitha K Rajaram, PhD,
Susan F Laska, MS,
Susan J Essenmacher,
Taichun Qin, PhD,
Tamala P Bogan,
Tamala P Magee,
Tamika White,
Tara A Gray,
Terri E Gibson,
Terri L Dodds,
Thanh M Andrews,
Thao T Kwan,
Thao X Tran,
Theresa Kirkham (NMI),
Thilak K Mudalige,
Thomas A Peter,
Thomas J Arista,
Thomas O Morgan,
Timothy C Grome,
Toby Vern H Hill,
Torrance J Slayton,
Traci C Kelm,
Tracy J Washington,
Travis M Beard,
Travis S Brown,
Tricia S Martinez,
Trina K Vick,
Truong Xuan Nguyen (Andy),
Tuan A Nguyen,
Uttaniti Limchumroon (Tom),
Vickie L Anderson,
Vicky L Cruz,
Victor Spanioli,
Virgilio F Pacio, CSO,
Vivian Garcia,
Walden H Lee,
Wanda B Coats,
William D Bassett, Jr,
William H Shackelford,
William Regnault, PhD,
Wr Bowman,
Xiaohui Shen,
Xiaojun Yan,
Yehualashet A Gessesse,
Young M Yoon,
Yumi J Hiramine,
Yvette E Guillermo,
Yvette Mlacour Davis,
Yvonne C Wilkes,
Yvonne Y Wu,
Zerita White
Iris C Macinnes's Documents
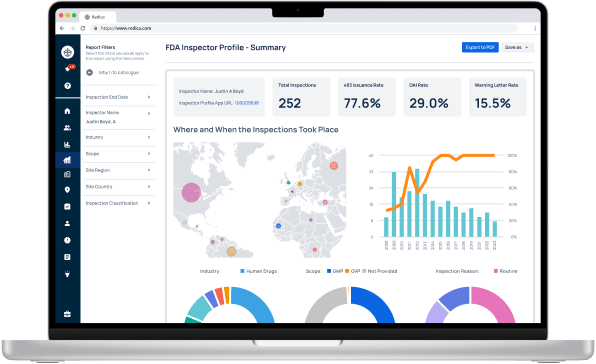
Experience Redica Systems’ NEW investigator profiles and dashboards
Redica Systems customers get access to over 1,400 FDA Investigator profiles, containing powerful analysis like:
- Total inspections conducted
- 483 rate
- OAI rate
- Warning Letter rate
- Inspections by Industry, Scope, and Inspection Reason
- And much more
Investigator Profiles are not sold as on-demand documents. If you’d like to see one, fill out the form below to connect with our Sales Team and get it in your inbox as little as 24 hours.
Talk to sales to access our investigator profiles
Loading...
Investigator Profiles Are Just the Tip of the Iceberg
Becoming a Redica Systems customer not only provides you with unlimited access to profiles similar to the one above for every current and former FDA Investigator, but also grants you access to:
Inspection Intelligence
- Pre-Approval Inspection (PAI) trends
- The ability to filter nearly any FDA enforcement action by GxP labels like GMP, GCP, etc.
- Latest 483s: see what the trends are across all FDA Investigators
- CFR Heatmap: Citations aggregated by Subpart by Year
Vendor Intelligence
- Full inspection histories for your vendors down to the specific site level
- All of the functionality mentioned above but isolated to just your vendors
Regulatory Intelligence
- Zero in on the trends for a specific agency/regulator (ex. FDA, MHRA, EMA, Health Canada)
- All the latest "Signals" from agencies around the world, segmented by Country, Type (ex. Guidance, Decree...), Category (ex. Rules/Regulations/Guidance, News, Reports...), Theme (ex. Cell and Gene Therapy, AI...), and more