FDA Investigator Karen L Kosar
Karen L Kosar has conducted inspections on 400 sites in 15 countries as of 05 Mar 2021. See below for a list of the FDA enforcement documents resulting from those inspections.
Investigator Details
Number of Inspected Sites:
400
Last Inspection Date:
05 Mar 2021
Investigator Role:
FDA Investigator
Redica ID:
Countries of Inspections:
United States of America,
Mexico,
Canada,
France,
United Kingdom of Great Britain and Northern Ireland,
Sweden,
Germany,
Italy,
China,
India,
Denmark,
Hungary,
Estonia,
Poland,
Dominican Republic
FDA Investigators that have inspected at least one site in common with Karen L Kosar:
Abby E Pelletier,
Alan L Truong,
Alia Legaux,
Alice S Tsao,
Althea A Williams,
Amal M Mansour,
Amanda T Zick,
Amatul H Marium,
Amber D Brodas,
Amy Kim,
Amy S Graf,
Ana P Pineda Zavaleta,
Anastasia M Shields,
Andrew B Paglia, DPM,
Andrew M Abramowitz,
Ann B Okon,
Anna Lazar,
Anthony E Keller, RPh,
Anthony Kovalenko,
Anthony N Onianwa,
Antonina Aydanian,
Arduino Frankovic,
Ariel Cruz Figueroa,
Arthur G Hurst,
Azza Talaat,
Benton M Ketron,
Bijoy Panicker,
Brandy N Lepage,
Brenda A Kraengel,
Brian J Ryan,
Bruce G Cooper,
Cara M Minelli,
Carl A Anderson,
Carolyn J Cook,
Cassandra L Winters,
Charanjeet Jassal,
Charisse K Green,
Charles Jewell,
Charles R Cote, RIC,
Cheryl L Manney,
Christian Parra,
Christopher S Fields,
Christopher S Keating,
Crystal A Branch,
Cynthia A Stempniak,
Daniel L Aisen,
David A Carlson,
David A Trent Carlson,
David J Eide,
David J Feria,
David J Leray,
David M Beltran,
David M Mcnew,
David R Delucia,
David R Lattuca,
Dean R Rugnetta,
Deborah A Greco,
Demitria J Argiropoulos,
Demitria J Xiradakis,
Denise L Terzian,
Dennis E Guilfoyle, PhD,
Diamond M Oden,
Diane E Bruce,
Diane L Raccasi,
Dianiris C Ayala,
Dipesh K Shah,
Donald G Gordon,
Donald J Ullstrom,
Douglas G Ng,
Dr. Abhijit Raha, PhD,
Dr. Jason R Caballero,
Dr. Robert C Horan, MD,
Dr. Sriram Subramaniam, PhD,
Elizabeth H Davis,
Elizabeth H Slazak,
Emest F Bizjak,
Erika E Englund, PhD,
Erin L Nagel,
Evelyn Taha,
Feiyan Jin,
Felix Maldonado,
Freyja V Lynn,
Gabriel Davila,
Gam S Zamil,
Gary J Lehr,
Gary T Greco,
George C Amedro,
Gregson A Joseph,
Guerlain Ulysse,
Harry J Brewer,
Helen B Ricalde,
Helen Verdel,
Hilary K Wagner,
Hoimay Chan,
Hugh M Mcclure, II,
Iram R Hassan, PhD,
Irina Gaberman,
Jacqueline Mdiaz Albertini,
Jacqueline S Warner,
James A Liubicich,
James C Maclaughlin,
James D Bridges,
James M Kewley,
James M Mason,
Janet A Rajan,
Janete F Guardia,
Jay B Shah,
Jay T Wong,
Jazmine N Brown,
Jeanette L Mcginnis,
Jeffery A Hangartner,
Jeffrey A Sommers,
Jennifer L Bridgewater,
Joanne M Schlossin,
Joel R Merriman,
Joey V Quitania,
John A Podsadowski,
John P Mistler,
Johnna L Bleem,
Jonah S Ufferfilge,
Jose Acruz Gonzalez,
Jose Ohernandez Guzman,
Jose R Hernandez,
Joseph A Famiglietti,
Joseph J Vannelli,
Joseph Maselli,
Joseph Milcetic,
Joseph T Frost,
Joshua D Lopez,
Joy P Matthias,
Joyce A Williams,
Juan Rjimenez Garcia,
Judy Keihl,
Julie Scoma,
Junho Pak,
Kara A Scheibner, PhD,
Karan L Blum,
Karen A Morgante,
Karen M Cooper,
Karen M Montgomery (KMM),
Katherine Szestypalow,
Kathryn A Nagy,
Kellie Leveille,
Kenneth G Klobus,
Kenneth H Williams,
Kerun Hardeo,
Kevin A Gonzalez,
Kim Lthomas Cruse,
Kim M Downing,
Kimberley A Gordon,
Kimberley A Ricketts,
Kimberly Y Smithson,
Kip J Hanks,
Kristina L Conroy,
Kyle J Spencer,
L Gnnmassimia Massimilla, PharmD,
Lata C Mathew, PhD,
Lauren E Blaser,
Laurie A Haxel,
Laurie B Frazier,
LCDR Chad N Thompson,
Leena Thomas,
Linda M Pyjas,
Linda M Sacco,
Lori Peters,
Lucas B Leake,
Luella J Rossi,
Marc S Neubauer,
Marcellinus D Dordunoo,
Margaret E Sarles,
Margaret M Annes,
Maria Estrella,
Maria Joselopez Barragan, PhD,
Marjorie A Shapiro, PhD,
Mark A Heard,
Marlene L Davis,
Marsha W Major,
Martha T Olone,
Mary Davis Lopez,
Mary M Finn,
Mary T Carden,
Mate Tolnay,
Matthew A Spataro,
Matthew B Casale,
Matthew D Schnittker,
Matthew W Kyle,
Mel Szymanski,
Melina Lrodriguez Upton,
Michael A Charles,
Michael F Skelly, PhD,
Michael G Sinkevich,
Michael Levin,
Michael P Sheehan,
Michael P Sweeney,
Michael R Dominick,
Michael Serrano,
Michael W Burd,
Michele L Obert,
Michele Perry Williams,
Michelle C Roberts,
Michelle M Noe Varga,
Miguel A Martinez Perez,
Mihaly S Ligmond,
Milan S Blake,
Mindy M Chou,
Miral B Patel,
Mohsen Rajabi Abhari, FDA,
Morris Paul,
Mra Davism,
Nancy L Neiger,
Nancy M Espinal,
Nancy T Waites,
Neil J Bonzagni, PhD MPH,
Nerizza B Guerin,
Nicholas C Mendiola,
Nicole M Bell,
Niketa Patel,
Nima Ossareh,
Noreen Muñiz,
Omadevi Somai, Investigator,
Omotunde O Osunsanmi,
Parul M Patel,
Patricia A Clark,
Patrick C Klotzbuecher,
Paul E Stein,
Paul Mouris,
Paula A Trost,
Paula J Bretz,
Perez,
Perry T Nichols,
Peter Abel,
Peter M Trunk,
Peter R Caparelli,
Peter S Diak,
Philip F Istafanos, DMV, MS,
Phyllis J Wilson,
Prabhu P Raju,
Rachael A Moliver,
Rafael Nevarez Nieves,
Rajiv R Srivastava,
Randi Lynn Bodoh,
Randi Lynn Spencer,
Rebecca Rodriguez,
Regina M Bennett,
Rene R Ramirez,
Rhoda B Eniafe,
Richard K Glabach,
Richard W Thornton,
Robert C Steyert,
Robert J Maffei,
Robert J Veitch,
Robert Jennings,
Roberto A Dookhan,
Rose Xu,
Russ E Davis,
Russell J Glapion,
Samuel T Walker,
Sandra A Hughes,
Sandra D Thompson,
Sangeeta M Khurana, PhD,
Santos E Camara,
Sarah A Meehan,
Sarah B Tanksley,
Sargum C Sood,
Sasha M Latonis,
Satheesh Thomas,
Sayyem H Akbar,
Scott E Norris,
Seongeun Cho (Julia), PhD,
Shannon A Dinardo,
Sherri J Jackson,
Shirley J Berryman,
Shreya Shah,
Simone E Pitts,
Sixto M Mercado Rios,
Sony Mathews,
Srinivas R Chennamaneni, PhD,
Sripal R Mada, PhD,
Stephen C Smith,
Steven E Bowen, PhD,
Steven J Libal,
Stuart W Russell,
Susan D Yuscius,
Susan F Laska, MS,
Susan M Jackson,
Susan W Ting,
Suyang Qin,
Syeda N Mahazabin,
Taichun Qin, PhD,
Tammy S Notto,
Tania E Vizcaino,
Tanya R Syffrard,
Tara K Carmody,
Tara K Normandin,
Tawny L Colling,
Thomas J Dinunzio,
Thomas J Mooney,
Thomas L Bellonte,
Thomas P Hansen,
Timothy T Kapsala,
Tina S Roecklein,
Tracy M Portelli,
Truong Xuan Nguyen (Andy),
Tyanna N Hadley,
Uduak M Inokon,
Vickie J Kanion,
Vien Q Le,
Vivin George,
Wayne E Seifert,
Wendy G Tan, PhD,
Wendy M Stone,
William C Lubas,
William J Leonard,
William M Rennells,
William P Chilton,
Xiaohan Cai, PhD,
Yiying E Chen,
Yiyue Zhang (nmi), PhD,
Yuyan Liang,
Yvesna C Blaise,
Yvins Dezan,
Yvonne C Mcknight,
Zhongren Wu
Karen L Kosar's Documents
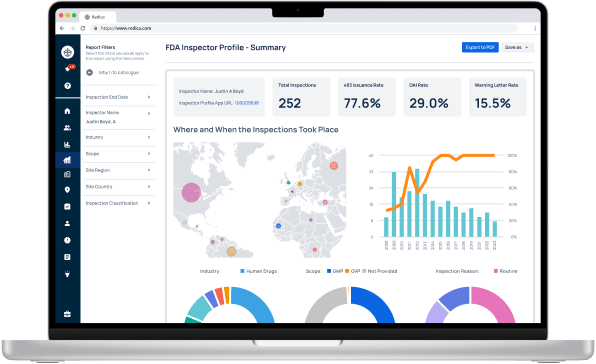
Experience Redica Systems’ NEW investigator profiles and dashboards
Redica Systems customers get access to over 1,400 FDA Investigator profiles, containing powerful analysis like:
- Total inspections conducted
- 483 rate
- OAI rate
- Warning Letter rate
- Inspections by Industry, Scope, and Inspection Reason
- And much more
Investigator Profiles are not sold as on-demand documents. If you’d like to see one, fill out the form below to connect with our Sales Team and get it in your inbox as little as 24 hours.
Talk to sales to access our investigator profiles
Loading...
Investigator Profiles Are Just the Tip of the Iceberg
Becoming a Redica Systems customer not only provides you with unlimited access to profiles similar to the one above for every current and former FDA Investigator, but also grants you access to:
Inspection Intelligence
- Pre-Approval Inspection (PAI) trends
- The ability to filter nearly any FDA enforcement action by GxP labels like GMP, GCP, etc.
- Latest 483s: see what the trends are across all FDA Investigators
- CFR Heatmap: Citations aggregated by Subpart by Year
Vendor Intelligence
- Full inspection histories for your vendors down to the specific site level
- All of the functionality mentioned above but isolated to just your vendors
Regulatory Intelligence
- Zero in on the trends for a specific agency/regulator (ex. FDA, MHRA, EMA, Health Canada)
- All the latest "Signals" from agencies around the world, segmented by Country, Type (ex. Guidance, Decree...), Category (ex. Rules/Regulations/Guidance, News, Reports...), Theme (ex. Cell and Gene Therapy, AI...), and more