FDA Investigator Richard W Berning
Richard W Berning has conducted inspections on 203 sites in 30 countries as of 27 May 2024. See below for a list of the FDA enforcement documents resulting from those inspections.
Investigator Details
Number of Inspected Sites:
203
Last Inspection Date:
27 May 2024
Investigator Role:
FDA Investigator
Redica ID:
Countries of Inspections:
United States of America,
Spain,
Japan,
New Zealand,
Israel,
Taiwan,
France,
Guinea,
Korea (Republic of),
United Kingdom of Great Britain and Northern Ireland,
Germany,
Brazil,
China,
Georgia,
India,
Czechia,
Sierra Leone,
Mexico,
Switzerland,
Romania,
Canada,
Austria,
Netherlands,
Liberia,
Serbia,
Italy,
Poland,
Australia,
Denmark,
Croatia
FDA Investigators that have inspected at least one site in common with Richard W Berning:
Ademola O Daramola,
Alan Escalona,
Alec M O'neal,
Allison C Hunter,
Allison E Sincek,
Allison M Scheck,
Amanda E Lewin, PhD,
Amanda J White,
Amy L Mccarthy,
Andrew I Carr,
Andrew J Lang,
Andrew M Kupper,
Andria L Kuhlman,
Anh M Lac,
Anita Narula, PhD,
Ann L Demarco,
Anna M Brannen,
Arie R Dyk,
Asad Ali,
Azza Talaat,
Bailey A Uetrecht,
Barbara D Wright,
Benjamin D Shuler,
Benjamin J Dastoli,
Benjamin P Stewart,
Benton M Ketron,
Bill Tacket, Jr,
Bo Chi, PhD,
Brenda C Hamilton,
Brenda W Uratani, PhD,
Brigitte K Hofmann,
Brittany M Kershaw,
Bryan J Love,
Carlos H Ruiz,
Cassandra L Winters,
Catherine D Djordjevic,
Ching Jeyg Chang,
Christian L Witkovskie,
Christina L Bigham,
Christine E Kelley,
Christopher D Snyder,
Christopher Downey, PhD,
Christopher T Middendorf,
Clinton D Priestley,
Concepcion Cruz, Jr,
Courtney N Long,
Craig A Garmendia,
Craig P Seaborn,
Craig T Rybus,
Cynthia F Kleppinger, MD,
Cynthia L Rakestraw,
Cynthia T Cain,
D'arbra R Blankenship,
Darren S Morgan,
David B Wieneke,
David L Chon,
David P Moorhead,
Dawn C Olenjack,
Dell S Moller,
Denise L Burosh,
Diane L Raccasi,
Diane M Fresch,
Diane R Mcdaniel,
Doan T Nguyen, PharmD,
Donald C Obenhuber, PhD,
Donna F Campbell,
Dorathy M Eischen,
Dr. Abhijit Raha, PhD,
Dr. Cj George Chang,
Dr. Gopa Biswas, PhD,
Dr. Mark J Seaton, PhD,
Dr. Ralph M Bernstein, PhD,
Dr. Sriram Subramaniam, PhD,
Dr. Zhihao Qiu (Peter), PhD,
Dr. Zhou Chen (nmi), MD PhD,
Dylan D Yao, MD, PhD,
Edward R Kay,
Eileen T Monaghan,
Eliezar Ramos,
Elizabeth Ll Edwards,
Elizabeth P Kinsella,
Emma A Nesbit,
Eric M Mueller, PharmD,
Erika V Butler,
Ethan P Stegman,
Evan M Kupper,
Farwa Z Razvi,
Felix Maldonado,
Frederick M Lochner,
Gam S Zamil,
Gene R Gunn,
Geoffrey K Kilili,
Gilbert J Heidenblut,
Gordon R Davidson,
Gretchen N Kilby,
Heather A Mccauley,
Holly J Rapier,
Holly J Wilson,
Hugh M Mcclure, II,
James G Marsh,
James M Kewley,
James P Mcevoy,
Janet L Bowen,
Janete F Guardia,
Jason Channels,
Jason D Werne,
Javonica F Penn,
Jeffrey A Sincek,
Jenn W Sellers,
Jennifer L Gustavus,
Jennifer L Jager,
Jennifer L Sheehan,
Jennifer L Watson,
Jennifer M Heitz,
Jerry J Corwin,
Jessica M Wooten,
Joanne M Schlossin,
Joel D Martinez,
John D Jaworski,
John D White,
John E Russell, Jr,
John P Roetting, II,
Jonathan A Ferguson,
Jonee J Mearns,
Joseph X Kaufman,
Joshua P Wireman,
Joshua S Hunt,
Julie D Bringger,
Kalavati Suvarna, PhD,
Karen M Cooper,
Karen M Kondas,
Kathleen D Culver,
Kathleen R Jones, PhD,
Kathryn Carroll,
Kathryn J Mccarty,
Kathryn Suttling,
Katie L Korchinski,
Keith J Mason, Jr,
Kelli L Wilkinson,
Kent A Conforti,
Kevin A Gonzalez,
Kham Phommachanh,
Kilili Kilili, Geoffrey K,
Kimberly A Joseph,
Kirk A Dymbrowski,
Krista B Jones,
Kurt A Brorson, PhD,
Lakecha N Lewis,
Lance Mde Souza, MBA,
Laura E Garcia,
Lauren N Smith,
Lauren R Collins,
Lesley K Satterwhite,
Lindsey M Schwierjohann,
Lisa A Warner,
Lisa M Marko,
Lori A Carr,
Lori A Gioia,
Luckner Jean Marie,
Makini Cobourne Duval, PhD,
Malik S Qaiser,
Maney P Sturgill,
Marcia A Worley,
Margaret Torres Vazquez,
Marianne Allen,
Maribeth G Niesen,
Mariza M Jafary,
Mark Brunswick, PhD,
Mark E Parmon,
Mary E Storch,
Matt D Suedkamp,
Matthew B Casale,
Melanie N Parsons,
Melkamu Getie Kebtie, PhD,
Meredith M Cobb,
Michael D Thatcher,
Michael E Campbell,
Michael G Sinkevich,
Michael K Larson,
Michael P Anthony,
Michael P Sheehan,
Michael Serrano,
Michael Shanks, MS,
Michelle Yclark Stuart,
Mihaly S Ligmond,
Milos Dokmanovic, PhD,
Min Lu,
Mishelle L Harriger,
Mohsen Rajabi Abhari, FDA,
Monica Commerford, PhD,
Mra Davism,
Mra Munizn,
Mra Narulaa,
Muralidhara B Gavini, PhD,
Nadeem I Chaudhry,
Nancy A Bellamy,
Nancy L Neiger,
Nicholas L Paulin,
Nilufer M Tampal, PhD,
Omotunde O Osunsanmi,
Pamela A Kuist,
Pankaj H Amin,
Parul M Patel,
Paul A Bonneau,
Payne,
Peter R Lenahan,
Philip S Woodward,
Phillip M Pontikos,
Pifer,
Prabhu P Raju,
Qing Joanna Zhou, PhD,
Raquel L Coleman,
Reyes Candau Chacon, PhD,
Richard Ledwidge (nmi), PhD,
Robbie J Alleman,
Robert Jennings,
Robert Rodriguez,
Rosanna M Goodrich,
Rose Ashley,
Roy Baby,
Roy C Stephens,
Rterry Bolen,
Samuel A Hamblin,
Scott B Laufenberg,
Sharon L Matson,
Sheri L Stephenson,
Sherri J Jackson,
Sripal R Mada, PhD,
Stephen J Kilker,
Steven P Eastham,
Stuart W Russell,
Susan D Yuscius,
Susan M Taylor,
Talmane J Fisher,
Tamara M Kays,
Tara K Carmody,
Tawny L Colling,
Teresa K Kastner,
Thomas B Smith,
Thomas C Woods,
Thomas J Prigge,
Thomas W Nojek,
Thunder N Dunkijacobs,
Thuy T Nguyen, LCDR,
Tracey L Harris,
Veronica Fuentes, MS,
Victoria M Daddeo,
Virgilio F Pacio, CSO,
Walter N Lange,
Wayne E Seifert,
Xikui Chen (nmi), PhD,
Yangmin Ning,
Yanming An, PhD,
Yiyue Zhang (nmi), PhD,
Yvette Mlacour Davis,
Zerita White,
Zhong Li, PhD,
Ziyang Su, PhD
Richard W Berning's Documents
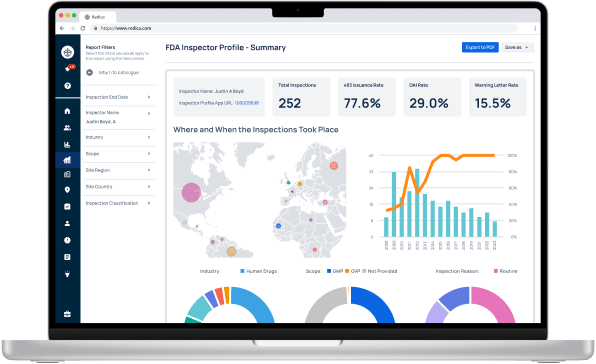
Experience Redica Systems’ NEW investigator profiles and dashboards
Redica Systems customers get access to over 1,400 FDA Investigator profiles, containing powerful analysis like:
- Total inspections conducted
- 483 rate
- OAI rate
- Warning Letter rate
- Inspections by Industry, Scope, and Inspection Reason
- And much more
Investigator Profiles are not sold as on-demand documents. If you’d like to see one, fill out the form below to connect with our Sales Team and get it in your inbox as little as 24 hours.
Talk to sales to access our investigator profiles
Loading...
Investigator Profiles Are Just the Tip of the Iceberg
Becoming a Redica Systems customer not only provides you with unlimited access to profiles similar to the one above for every current and former FDA Investigator, but also grants you access to:
Inspection Intelligence
- Pre-Approval Inspection (PAI) trends
- The ability to filter nearly any FDA enforcement action by GxP labels like GMP, GCP, etc.
- Latest 483s: see what the trends are across all FDA Investigators
- CFR Heatmap: Citations aggregated by Subpart by Year
Vendor Intelligence
- Full inspection histories for your vendors down to the specific site level
- All of the functionality mentioned above but isolated to just your vendors
Regulatory Intelligence
- Zero in on the trends for a specific agency/regulator (ex. FDA, MHRA, EMA, Health Canada)
- All the latest "Signals" from agencies around the world, segmented by Country, Type (ex. Guidance, Decree...), Category (ex. Rules/Regulations/Guidance, News, Reports...), Theme (ex. Cell and Gene Therapy, AI...), and more