FDA Investigator Jacqueline S Warner
Jacqueline S Warner has conducted inspections on 353 sites in 24 countries as of 22 Apr 2024. See below for a list of the FDA enforcement documents resulting from those inspections.
Investigator Details
Number of Inspected Sites:
353
Last Inspection Date:
22 Apr 2024
Investigator Role:
FDA Investigator
Redica ID:
Countries of Inspections:
United States of America,
China,
Canada,
Switzerland,
Germany,
India,
Norway,
Thailand,
France,
Italy,
Korea (Republic of),
Sweden,
United Kingdom of Great Britain and Northern Ireland,
Taiwan,
Brazil,
Ireland,
Denmark,
Austria,
Belgium,
Finland,
Singapore,
Japan,
Malaysia,
Spain
FDA Investigators that have inspected at least one site in common with Jacqueline S Warner:
Addam S Reynolds,
Adetutu M Gidado,
Affiong Flores,
Akbar J Zaidi,
Alex K Yan,
Alice S Tsao,
Alisha B Patel,
Allison M Scheck,
Almaris N Alonso,
Althea A Williams,
Amanda S Zorn,
Amber D Brodas,
Amber D Nair,
Amy M Cramer,
Amy S Graf,
Amy Wyan Mai,
Ana S Cabrera,
Anastasia M Shields,
Andrew F Cohen,
Andrew J Garufi,
Anissa M Vargas,
Ann B Okon,
Anthony M Criscuolo, Jr,
Antonina Aydanian,
Arduino Frankovic,
Ariel Cruz Figueroa,
Arsen Karapetyan,
Arthur S Williams, Jr,
Ashleigh P Barkans,
Ashleigh P Wodushek,
Ashley A Mutawakkil,
Ashley Polizzotto,
Benjamin J Dastoli,
Bonnie E Conley,
Brenna L Macdowell,
Brian R Yaun,
Brian S Keefer,
Brittani N Franklin,
Brooke K Seeman,
Bryan J Love,
Byungja E Marciante,
Carolyn J Cook,
Caryn M Mcnab,
Catherine J Laufmann,
Catherine M Beer,
CDR Sean T Creighton,
Charanjeet Jassal,
Charisse K Green,
Charles J Chacko,
Charles Jewell,
Charles L Larson,
Cheryl A Clausen,
Christian Parra,
Christine I Shaw,
Christine M Parmentier,
Christine M Rivera,
Christopher A Demitrius,
Christopher J Adams,
Christopher S Fields,
Concepcion Cruz, Jr,
Creighton T Tuzon,
Cynthia Jim, CSO,
Cynthia T Cain,
Daniel J Grabicki,
Dave M Deroche,
David H Smith,
David P Vanhouten,
David R Delucia,
Dawn L Wydner,
Dawn M Braswell,
Deborah A Greco,
Demitria J Argiropoulos,
Demitria J Xiradakis,
Denise L Terzian,
Dennis E Guilfoyle, PhD,
Dennis R Hock,
Devon O Seymour,
Diane L Raccasi,
Dipesh K Shah,
Dolores Harper,
Donald G Gordon,
Doreen P Gubbay,
Dorothy W Lee,
Douglas A Campbell,
Douglas C Kovacs,
Douglas G Ng,
Dr. Robert C Horan, MD,
Duo D Chen,
Edouard Viard,
Edward D Mcdonald,
Edward J Janik,
Edward R Kay,
Elizabeth D Connell,
Ellen J Tave,
Ellen M Landis,
Elvin R Smith,
Emily A Walters,
Emmanuel A Dimaano, Jr,
Eric A Mann,
Eric S Pittman,
Erica B Brush,
Erica B Raphael,
Eufemia G Gonzalez,
Evelyn Taha,
Fatima Bassa,
Felix J Marrero,
Felix Maldonado,
Francis A Guidry,
Francis J Eng,
Frank J Marciniak,
Frank Verni,
Freyja V Lynn,
Gale E Ryan,
Gam S Zamil,
Gary L Pierce,
Gene D Arcy,
Geoffrey K Kilili,
George G Calafactor,
Gerald D Bromley, Jr,
German Rivera,
Gifford Whitehurst, Jr,
Grace E Mcnally,
Gregson A Joseph,
Helen B Ricalde,
Helen Verdel,
Iram R Hassan, PhD,
Iris C Macinnes,
Ivis L Negron,
J Elkins,
Jackson Y See,
Jacqueline Mdiaz Albertini,
James A Beaulieu,
James A Liubicich,
James C Maclaughlin,
James D Hildreth,
James D Planchon,
James K Kane, PhD,
James L Finn,
James M Odonnell,
James R Birkenstamm,
James R Montero,
Jane C Chen,
Janet Pulver,
Janete F Guardia,
Janice A Hawkins,
Jay B Shah,
Jay T Wong,
Jean M Kelahan,
Jeffrey J Thibodeau,
Jennifer A Bazergui,
Jennifer L Bridgewater,
Jennifer L Custodio,
Jennifer Macmillan,
Jennifer S Ness,
Jesse A Vazquez,
Jocelyn C Turner,
Jocelyn E Massey,
Jocelyn E Sparks,
Jogy George,
John A Sciacchitano,
John E Aghadia,
Jonathan Ho,
Jose Ohernandez Guzman,
Joseph D Gong,
Joseph Maselli,
Joseph Milcetic,
Joseph R Strelnik,
Joseph S Tomao,
Joy P Matthias,
Juan Rjimenez Garcia,
Juanita P Versace,
Judy Friedman,
Judy Keihl,
Juliana Falla,
Junho Pak,
Justine Tomasso,
Karen Emasley Joseph,
Karen L Kosar,
Karishma G Gopaul,
Kelli F Dobilas,
Kellia N Hicks,
Kelly I Anderson,
Kelly L Anderson,
Kenneth H Williams,
Kerry A Kurdilla,
Kerun Hardeo,
Kevin A Gonzalez,
Kham Phommachanh,
Kimberley A Gordon,
Kimberley A Ricketts,
Kimberly Lewandowski Walker,
Kinh Q Mac,
Kristen C Jackson,
Kristina L Conroy,
Kristy A Zielny,
Kwong P Lee,
L Gnnmassimia Massimilla, PharmD,
Larry K Austin,
Lata C Mathew, PhD,
Laureen M Geniusz,
LCDR Chad N Thompson,
LCDR Matthew J Morrison,
LCDR Wilfred A Darang,
Leena Thomas,
Leo J Lagrotte,
Leonard H Lavi,
Li Li,
Linda A Gregory Duty,
Lirissia Mccoy,
Lisa A Warner,
Lisa M Lopez,
Lisa Mathew,
Lori Peters,
LT Colin E Tack, BSE,
LTJG Matthew R Palo,
Marcelo O Mangalindan, Jr,
Margaret E Sarles,
Margaret M Sands,
Maria Joselopez Barragan, PhD,
Maricelys Mercado,
Marie Elena C Puleo,
Marjorie A Shapiro, PhD,
Mark E Chan,
Marlo Ianm Alintanahin,
Marsha W Major,
Martha T Olone,
Martina E Lagrange,
Mary A Millner,
Mary Davis Lopez,
Mary M Finn,
Maryam Tabatabaie,
Mate Tolnay,
Matthew A Spataro,
Matthew D Schnittker,
Matthew J Sienko,
Matthew W Kyle,
Maura Rooney,
Meisha A Mandal,
Melanie G Warzala,
Melanie W Pishnery,
Melissa A Henaghan,
Melissa J Garcia,
Melissa N Gregorio,
Mercedes G Castillo,
Meredith L Sheridan,
Meredith P Soehl, Investigator,
Michael A Charles,
Michael A Taylor,
Michael G Mayfield,
Michael G Sinkevich,
Michael J Nerz,
Michael K Larson,
Michael R Dominick,
Michael Serrano,
Michele L Obert,
Michelle J Glembin,
Mihaly S Ligmond,
Mikayla K Deardorff,
Milan S Blake,
Milan S Mcgorty,
Mindy M Chou,
Mra Mcculloughj,
Naakesh N Gomanie,
Nancy A Saxenian Emmons,
Nancy T Waites,
Nerizza B Dalena,
Nerizza B Guerin,
Nicholas C Mendiola,
Nicole M Li,
Norman Wong,
Nur M Faisal,
Omotunde O Osunsanmi,
Parul M Patel,
Patricia A Clark,
Paul E Stein,
Paul L Bellamy,
Paul Mouris,
Paul R Whitby,
Paula A Trost,
Pearl L Gonzalez,
Perez,
Perry H Gambrell,
Perry T Nichols,
Peter Abel,
Peter M Trunk,
Peter R Caparelli,
Philip F Istafanos, DMV, MS,
Prabhu P Raju,
Preston M Lee,
Puspa Suresh Jayasekara,
Rachael A Moliver,
Rachael O Oyewole,
Raffi B Papazian,
Rajiv R Srivastava,
Ramon A Hernandez,
Raymond M Lam,
Rebecca Rodriguez,
Regina T Brown,
Richard A Lewis,
Richard Jay Tucker, RS,
Richard K Glabach,
Richard K Vogel,
Richard L Rutherford,
Richard W Thornton,
Rita F Monfort,
Robert C Steyert,
Robert E Moncayo,
Robert G Antonsen, Jr,
Robert G Ruff,
Robert Jennings,
Rochelle K Kimmel,
Rodney D Combs,
Ronald Ifraimov,
Rosanna M Goodrich,
Rose Ljean Mary,
Roy Baby,
Russell K Riley,
Salvatore N Randazzo,
Samuel A Francis,
Samuel T Walker,
Sarah A Hassas,
Sarah A Meehan,
Sarah B Tanksley,
Sarah Forney,
Sargum C Sood,
Satheesh Thomas,
Scott E Norris,
Scott R Izyk,
Selene T Torres,
Sergio Chavez (NMI),
Shafiq S Ahadi,
Shelina Parvin,
Simone E Pitts,
Sonia E Ortiz,
Sony Mathews,
Sripal R Mada, PhD,
Stanley Noneze,
Stephanie T Durso,
Stephen C Smith,
Stephen D Brown,
Stephen D Eich,
Stephen R Souza,
Steven M Weinman,
Steven R Ziser,
Susan A Zullo, PhD,
Susan F Laska, MS,
Susan M Jackson,
Susan T Hadman,
Susan W Ting,
Suwaskie S Smith,
Syeda N Mahazabin,
Taina Pierre Pierre,
Tamil Arasu, PhD,
Tania E Garcia,
Tania E Vizcaino,
Tara K Carmody,
Tara K Normandin,
Terry C Kimball,
Thai T Duong,
Thomas J Mooney,
Thomas P Hansen,
Thomas Siebertz,
Tina S Roecklein,
Torrance J Slayton,
Tracy M Portelli,
Uduak M Inokon,
Valentino Fiorella,
Valerie A Potopsingh,
Vanessa M Williams,
Vincent T Duncan,
Vivin George,
Wanda J Torres,
Wayne E Seifert,
Wendy M Stone,
William D Bassett, Jr,
William M Rennells,
William R Brubaker,
William Regnault, PhD,
William V Thompson,
Winston R Alejo,
Yehualashet A Gessesse,
Youkeun Kim,
Yumi J Hiramine,
Yuyan Liang,
Yvette Mlacour Davis,
Yvins Dezan,
Yvonne M Santiago
Jacqueline S Warner's Documents
Experience Redica Systems’ NEW investigator profiles and dashboards
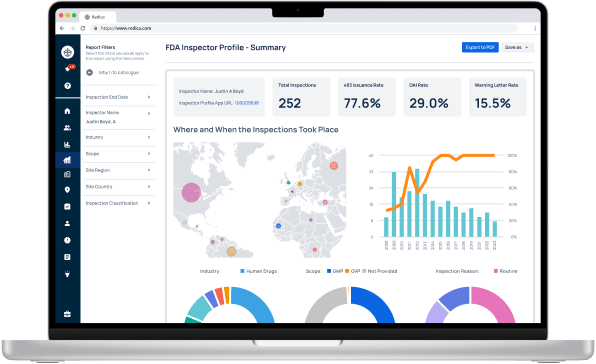
Redica Systems customers get access to over 1,400 FDA Investigator profiles, containing powerful analysis like:
- Total inspections conducted
- 483 rate
- OAI rate
- Warning Letter rate
- Inspections by Industry, Scope, and Inspection Reason
- And much more
Investigator Profiles are not sold as on-demand documents. If you’d like to see one, fill out the form below to connect with our Sales Team and get it in your inbox as little as 24 hours.
Talk to sales to access our investigator profiles
Loading...
Investigator Profiles Are Just the Tip of the Iceberg
Becoming a Redica Systems customer not only provides you with unlimited access to profiles similar to the one above for every current and former FDA Investigator, but also grants you access to:
Inspection Intelligence
- Pre-Approval Inspection (PAI) trends
- The ability to filter nearly any FDA enforcement action by GxP labels like GMP, GCP, etc.
- Latest 483s: see what the trends are across all FDA Investigators
- CFR Heatmap: Citations aggregated by Subpart by Year
Vendor Intelligence
- Full inspection histories for your vendors down to the specific site level
- All of the functionality mentioned above but isolated to just your vendors
Regulatory Intelligence
- Zero in on the trends for a specific agency/regulator (ex. FDA, MHRA, EMA, Health Canada)
- All the latest "Signals" from agencies around the world, segmented by Country, Type (ex. Guidance, Decree...), Category (ex. Rules/Regulations/Guidance, News, Reports...), Theme (ex. Cell and Gene Therapy, AI...), and more