FDA Investigator Stacie A Woods
Stacie A Woods has conducted inspections on 31 sites in 10 countries as of 26 Jun 2017. See below for a list of the FDA enforcement documents resulting from those inspections.
Investigator Details
Number of Inspected Sites:
31
Last Inspection Date:
26 Jun 2017
Investigator Role:
FDA Investigator
Redica ID:
Countries of Inspections:
Greece,
India,
United Kingdom of Great Britain and Northern Ireland,
United States of America,
China,
Canada,
Macao,
Puerto Rico,
Brazil,
Singapore
FDA Investigators that have inspected at least one site in common with Stacie A Woods:
Alberto A Viciedo,
Alexandra B Pitkin,
Anastasia M Shields,
Andrea A Branche,
Annet R Rajan,
Anthony C Warchut,
Anthony J Donato,
Arie C Menachem,
Ariel Cruz Figueroa,
Arlene M Badillo,
Arsen Karapetyan,
Ashar P Parikh,
Azza Talaat,
Barbara M Frazier,
Binh T Nguyen,
Brian D Nicholson,
Brian J Ryan,
Brian P Putz,
Brooke K Higgins,
Bush,
Camerson E Moore,
Carla J Lundi,
Carol L Rehkopf,
Caryn M Mcnab,
CDR Ileana Barreto Pettit,
Celena Ngo,
Charisse K Green,
Christopher D Washington,
Christopher N Tran,
Cynthia J Lee, MS,
Daniel J Lahar,
Darrin E Davis,
David M Beltran,
David Perkins,
Davis,
Dina K West,
Djamila Harouaka,
Doan T Nguyen, PharmD,
Dogbeda F Mackenzie,
Donald C Obenhuber, PhD,
Dr. Chunchang Fang,
Ellen J Kleintop,
Ellen J Tave,
Emest F Bizjak,
Felix Maldonado,
Gale E Ryan,
Gary R Wong,
Gayle S Lawson,
George J Flynn,
German Rivera,
Greg K Keshishyan,
Gunneet Kaur,
Gwyn G Dickinson,
Haijing Hu,
Haroon Vohra (NMI),
Henry E Carrillo,
Ivis L Negron,
Jai P Singh,
James C Maclaughlin,
Janet A Rajan,
Jawaid Hamid,
Jeffrey P Raimondi,
Jessica L Pressley,
Joan A Loreng,
Joey V Quitania,
John A Gonzalez,
John P Mistler,
Jolanna A Norton,
Jonathan G Matrisciano,
Jonathan T Little,
Jonathan W Chapman,
Jorge L Guadalupe,
Jose A Lopez,
Jose A Moreno,
Jose Acruz Gonzalez,
José E Meléndez,
Jose F Pedro,
Jose M Cayuela,
Jose Martinez, Jr,
Jose R Hernandez,
Jose R Lopez,
Juanita Banuelos,
Judy C Nepsa,
Justin A Boyd,
Karen K Moksnes,
Karen Takahashi,
Kari M Johansen,
Katherine E Jacobitz,
Katherine Szestypalow,
Keeshunna D Daniels,
Kelley,
Kelley M Simms,
Kellia N Hicks,
Kelvin X Sanders,
Kimberly Jproctor Jones,
Kouros Kangarli,
Kristin M Abaonza,
Lakecha N Lewis,
Lance A Finnical,
Larry K Austin,
Lata C Mathew, PhD,
Laurimer Kuilan Torres,
Leonard H Lavi,
Lilly O Barton,
Linda F Murphy,
Linda Thai,
Lisa R Whitt,
Lori M Graham,
Luis Mburgos Medero,
Lydia I Rosas Marty,
Margaret M Sands,
Maria Estrella,
Marianela Aponte Cruz,
Marie A Fadden,
Mark C Flotte,
Matthew B Casale,
Maxyne T Lam,
Melinda L Rice,
Michael A Charles,
Michael D Garcia,
Michael J Nerz,
Miguel A Martinez Perez,
Mindy M Chou,
Monique Rac Lester,
Mra Mcculloughj,
Muralidhara B Gavini, PhD,
Neal L Adams,
Nebil A Oumer,
Niketa Patel,
Patricia F Hughes, PhD,
Patty P Kaewussdangkul,
Paul L Bellamy,
Paul M Kawamoto,
Peter E Baker,
Qin Xu,
Rachel C Harrington,
Rebecca Parrilla,
Rebecca Rodriguez,
Richard H Penta,
Richmond K Yip,
Rochelle K Kimmel,
Ross J Grigsby,
Rumany C Penn, PharmD,
Ryan J Borges,
S Lori Brown, PhD MPH,
Saied A Asbagh,
Sam Pepe,
Samuel T Walker,
Sandra A Hughes,
Satheesh Thomas,
Sixto M Mercado Rios,
Sonia R Peterson,
Sony Mathews,
Stephanie A Slater, MS,
Stephen D Brown,
Steven E Porter, Jr,
Sundy V Sedwick,
Susan W Ting,
Tajah L Blackburn,
Tamala P Bogan,
Tamala P Magee,
Thao T Kwan,
Tiara Nbrown Crosen,
Tonya R Johnson,
Toyin B Oladimeji,
Truong Xuan Nguyen (Andy),
Uduak M Inokon,
Unnee Ranjan,
Uttaniti Limchumroon (Tom),
Walden H Lee,
William J Leonard,
Wr Bowman,
Yen Tso Kuo,
Yumi J Hiramine,
Zachary A Bogorad
Stacie A Woods's Documents
| Publish Date | Document Type | Title |
|---|---|---|
| June, 2017 | FDA 483 | MSN Life Sciences Private Limited - Form 483, 2017-06-30 |
| July, 2015 | FDA 483 | Iso-Tex Diagnostics, Inc. - Form 483, 2015-07-10 |
| February, 2017 | FDA 483 | Boehringer Ingelheim Animal Health do Brasil Ltda - Form 483, 2017-02-07 |
| February, 2017 | FDA 483 | BioVectra Inc - Form 483, 2017-02-17 |
| August, 2016 | EIR | SPECTRUM LABORATORY PRODUCTS INC. dba SPECTRUM CHEMICAL MFG. CORP. - EIR, 2016-08-25 |
| April, 2016 | FDA 483 | RX South DBA RX3 Compounding Pharmacy LLC - Form 483, 2016-04-18 |
| May, 2017 | FDA 483 | The Wellness Pharmacy - Form 483, 2017-05-16 |
| January, 2016 | FDA 483 | kdc/one Chatsworth, Inc. - Form 483, 2016-01-08 |
| December, 2015 | FDA 483 | DLC Laboratories, Inc. - Form 483, 2015-12-11 |
| March, 2017 | FDA 483 | MedPark Pharmacy, LLC - Form 483, 2017-03-10 |
| February, 2016 | FDA 483 | Central Admixture Pharmacy Serv - Form 483, 2016-02-05 |
| June, 2017 | FDA 483 | Dr Reddy's Laboratories Limited - Form 483, 2017-06-16 |
Experience Redica Systems’ NEW investigator profiles and dashboards
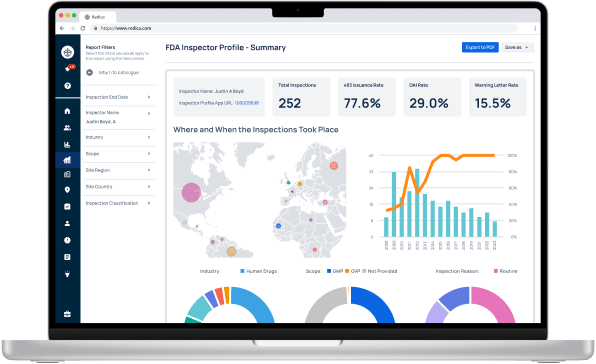
Redica Systems customers get access to over 1,400 FDA Investigator profiles, containing powerful analysis like:
- Total inspections conducted
- 483 rate
- OAI rate
- Warning Letter rate
- Inspections by Industry, Scope, and Inspection Reason
- And much more
Investigator Profiles are not sold as on-demand documents. If you’d like to see one, fill out the form below to connect with our Sales Team and get it in your inbox as little as 24 hours.
Talk to sales to access our investigator profiles
Loading...
Investigator Profiles Are Just the Tip of the Iceberg
Becoming a Redica Systems customer not only provides you with unlimited access to profiles similar to the one above for every current and former FDA Investigator, but also grants you access to:
Inspection Intelligence
- Pre-Approval Inspection (PAI) trends
- The ability to filter nearly any FDA enforcement action by GxP labels like GMP, GCP, etc.
- Latest 483s: see what the trends are across all FDA Investigators
- CFR Heatmap: Citations aggregated by Subpart by Year
Vendor Intelligence
- Full inspection histories for your vendors down to the specific site level
- All of the functionality mentioned above but isolated to just your vendors
Regulatory Intelligence
- Zero in on the trends for a specific agency/regulator (ex. FDA, MHRA, EMA, Health Canada)
- All the latest "Signals" from agencies around the world, segmented by Country, Type (ex. Guidance, Decree...), Category (ex. Rules/Regulations/Guidance, News, Reports...), Theme (ex. Cell and Gene Therapy, AI...), and more