FDA Investigator Frank J Marciniak
Frank J Marciniak has conducted inspections on 359 sites in 28 countries as of 09 Apr 2024. See below for a list of the FDA enforcement documents resulting from those inspections.
Investigator Details
Number of Inspected Sites:
359
Last Inspection Date:
09 Apr 2024
Investigator Role:
FDA Investigator
Redica ID:
Countries of Inspections:
United States of America,
Switzerland,
Guernsey,
Canada,
United Kingdom of Great Britain and Northern Ireland,
China,
Japan,
Austria,
Ireland,
France,
Czechia,
Italy,
Germany,
Taiwan,
Denmark,
Malaysia,
Korea (Republic of),
Costa Rica,
Spain,
Netherlands,
Philippines,
Finland,
Slovakia,
Benin,
Mexico,
Uruguay,
Thailand,
New Zealand
FDA Investigators that have inspected at least one site in common with Frank J Marciniak:
Aaron L Dunbar,
Adam S Freeman,
Addam S Reynolds,
Adetutu M Gidado,
Akbar J Zaidi,
Alberto A Viciedo,
Alexandra A Carrico,
Alice S Tsao,
Allen Lou,
Alois P Provost,
Althea A Williams,
Amanda Dinaro,
Amy A Johnson,
Amy L Singer,
Amy M Cramer,
Ana S Cabrera,
Ankur C Patel,
Ann Marie Montemurro,
Annemarie B Randow,
Annemarie Bodnar,
Annet R Rajan,
Anthony J Donato,
Anthony M Criscuolo, Jr,
Arthur S Williams, Jr,
Ashley J Burns,
Atul J Agrawal,
Barbara J Maulfair,
Barbara Jwilimczyk Macri,
Benjamin J Dastoli,
Bill Tacket, Jr,
Billi Jom Johnson,
Brenda L Reihing,
Brian S Keefer,
Brigitte K Hofmann,
Brigitte Kh Strelnik,
Burnell M Henry,
Byungja E Marciante,
Carl A Perez,
Cary Greene,
Catherine J Laufmann,
CDR Sean T Creighton,
Charles J Chacko,
Charles L Larson,
Cheryl D Mccall,
Christian D Lynch (CDL),
Christina N Maurino,
Christine M Cerenzio,
Christopher J Adams,
Claudette D Brooks,
Craig W Swanson,
Creighton T Tuzon,
Cynthia Jim, CSO,
Cynthia L Gorveatt,
Cynthia R Gibson,
Daniel J Grabicki,
Daniel J Lahar,
Daniel L Aisen,
Daniel S Fam,
Darla J Christopher,
Dave M Deroche,
David H Smith,
David J Gasparovich,
David J Gomes,
David M Wilkinson,
David P Vanhouten,
Dawn L Wydner,
Dawn M Braswell,
Deborah A Nebenzahl,
Deborah B Nixon,
Denise M Visco, Investigator,
Dennis E Guilfoyle, PhD,
Dennis G Kawabata,
Dhaval H Patel,
Diane B Radice,
Dianiris C Ayala,
Dipesh K Shah,
Dolores Harper,
Donna Ltartaglino Besone,
Doreen P Canetti,
Doreen P Gubbay,
Dorothy J Denes,
Douglas C Kovacs,
Dr. Guang Gao, PhD,
Edmund F Mrak, Jr,
Edward D Mcdonald,
Edward O'shaughnessy,
Eileen A Liu,
Elias N Paz Alonzo,
Eliezar Ramos,
Elizabeth D Connell,
Emest F Bizjak,
Emily A Walters,
Emmanuel Jramos Maldonado,
Eric J Cunningham,
Eric M Padgett,
Eric Rothschild,
Eric S Pittman,
Erica M Katherine,
Erin D Mccaffery,
Erin L Mcfiren,
Esteban Beltran,
Evelyn Taha,
Felix J Marrero,
Francis J Eng,
Frederick F Razzaghi,
Gail L Katz,
Gamal A Norton,
Gene D Arcy,
George T Allen, Jr,
Gerald D Bromley, Jr,
Gianine E Delade,
Gobiga Vanniyasingam,
Gregson A Joseph,
Guerlain Ulysse,
Harry J Brewer,
Helen B Ricalde,
Helen Verdel,
Jacqueline Mdiaz Albertini,
Jacqueline S Warner,
James E Frye,
James H Robinson,
James M Odonnell,
James M Simpson,
James P Mcreavey,
James R Birkenstamm,
James R Montero,
James W Plucinski,
Jane R Donnelly,
Janet B Abt,
Janet Pulver,
Janete A Eaker,
Janete A Oliveira,
Janete F Guardia,
Janice L King,
Jason M Sluzynski,
Javonica F Penn,
Jean M Kelahan,
Jennifer A Bazergui,
Jennifer L Custodio,
Jennifer M Menendez,
Jennifer Macmillan,
Jesse A Vazquez,
Jessica M Monteiro,
Jessica S Estriplet,
Jocelyn C Turner,
Jocelyn E Massey,
Jocelyn M Muggli,
Jogy George,
John A Sciacchitano,
John M Seale,
Jonathan B Lewis,
Jonathan Ho,
Jonee J Mearns,
Jose M Cayuela,
Joseph Duca,
Joseph F Mcginnis, RPh,
Joseph L Despins, PhD,
Joshua C Schafer,
Joshua J Silvestri,
Joy F Canlas,
Joy Rkozlowski Klena,
Judy Strojny Not In Use,
Julie D Bringger,
Justine Tomasso,
Karen A Briggs,
Karen A Coleman,
Karen E D'orazio,
Karen F Tomaziefski,
Karen M Rodriguez,
Karen S Anthony,
Katherine E Jacobitz,
Kazi Rafiquzzaman,
Keith M Reilly,
Kelli F Dobilas,
Kelli F Regenye,
Kelly Doremus,
Kelly I Anderson,
Kelvin Cheung,
Kerry A Kurdilla,
Kevin N Tran,
Kham Phommachanh,
Kinh Q Mac,
Kish Bolden,
Kristen E Rescigno,
Kristy A Zielny,
Ladislav Kermet,
Laishan L Lam,
Laureen M Geniusz,
Lauren L Vajo,
Lauren M Lawrance,
Lauren M Mclaughlin,
Laurie A Haxel,
Laurie B Frazier,
Laurimer Kuilan Torres,
Lawrence R Johnson,
LCDR Debra Emerson,
LCDR Wilfred A Darang,
Leo J Lagrotte,
Leonard H Lavi,
Leslie R Clark,
Li Li,
Liatte Kreuger, PharmD,
Linda A Gregory Duty,
Linda S Mattingly,
Lisa B Hall,
Lisa Harlan,
Lisa Mathew,
Loretta Nemchik,
Lori A Carr,
LT Colin E Tack, BSE,
LT Tamara J Henderson,
Lydia S Chan,
Malik S Qaiser,
Marcellinus D Dordunoo,
Marcelo O Mangalindan, Jr,
Marea K Harmon,
Margaret M Sands,
Maria Estrella,
Marjorie D Schultz,
Mark E Chan,
Martha T Olone,
Maryam Tabatabaie,
Matthew D Schnittker,
Matthew W Kyle,
Mei Chen Mu,
Meihong Liu,
Melanie W Pishnery,
Melba Trivera Clavell,
Melissa A Freeman,
Melissa A Zuppe,
Melissa B Libby,
Melissa T Roy,
Melka F Argaw,
Meredith L Sheridan,
Michael A Taylor,
Michael J Nerz,
Michael R Klapal,
Michael Rosner,
Michael Serrano,
Michele Gottshall,
Michelle J Glembin,
Michelle L Johnson,
Michelle M Parisi,
Miguel Gmanzano Maldonado,
Mihaela Jason,
Mike M Rashti,
Mishelle L Harriger,
Mizanne E Lewis,
Monica L Gutierrez,
Monika Borkowska,
Naakesh N Gomanie,
Nadeem I Chaudhry,
Nancy F Scheraga,
Nancy L Neiger,
Nancy L Rolli,
Nancy M Espinal,
Nathaniel R Esaw,
Nerizza B Guerin,
Nicholas A Violand,
Nicholas C Mendiola,
Niketa Patel,
Nikki S Ramirez,
Nisha C Patel,
Nydia E Colon,
Omotunde O Osunsanmi,
Paige E Shelborne,
Paige E Wilson,
Paige R Mccoy,
Parul M Patel,
Paul E Stein,
Paul L Bellamy,
Paul R Whitby,
Paula A Trost,
Pauline N Logan,
Perry H Gambrell,
Peter R Lenahan,
Phares O Okelo,
Phillip M Pontikos,
Prabhu P Raju,
Raffi B Papazian,
Ralph Jerndal,
Raymond L Cheung,
Regina T Brown,
Remache,
Richard D Manney,
Richard Jay Tucker, RS,
Richard L Rutherford,
Richard T Riggie,
Robert G Ruff,
Robert S Sweeton,
Ronald Ifraimov,
Rosanna M Goodrich,
Rose Ashley,
Rose Ljean Mary,
Roy Baby,
Russell J Glapion,
Saleem A Akhtar,
Sandra Kershaw,
Sarah A Hassas,
Sarah Forney,
Sargum C Sood,
Schultz,
Scott R Izyk,
Seema S Singh,
Selene T Torres,
Shafiq S Ahadi,
Sharmila Shrestha,
Sherri J Liu,
Sherry G Bous,
Shirley S Wen,
Simone E Pitts,
Sinai I Davis,
Stanley B Eugene, BS, BME,
Stephanie T Durso,
Stephen C Smith,
Stephen D Eich,
Stephen J Mottola,
Stephen R Souza,
Stephenie M Ortiz,
Steven E Kane,
Steven M Weinman,
Steven R Ziser,
Steven S Wahl,
Susan M Jackson,
Susan M Matthias,
Tania E Garcia,
Tania E Vizcaino,
Tara G Bizjak,
Tara R Gooen,
Teresa Jimenez,
Teresita C Mercado,
Thai T Duong,
Thomas A Peter,
Thomas E Friel,
Thomas J Arista,
Tonia F Bernard,
Tony T Yang,
Tracey L Harris,
Travis S Bradley,
Tressa T Lewis,
Valerie C Reed,
Valerie Reed,
Victor Spanioli,
Vivian Garcia,
Wei Wang, PhD,
Wendy M Stone,
Wendy R Blame,
William R Chang,
Xiaojun Yan,
Xiaoping Guan,
Xiuju Lu,
Yehualashet A Gessesse,
Yung W Chan,
Yvesna C Blaise,
Yvette Mlacour Davis,
Yvins Dezan,
Zakaria I Ganiyu
Frank J Marciniak's Documents
Experience Redica Systems’ NEW investigator profiles and dashboards
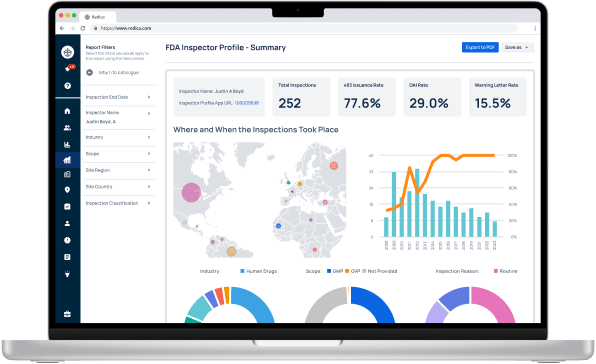
Redica Systems customers get access to over 1,400 FDA Investigator profiles, containing powerful analysis like:
- Total inspections conducted
- 483 rate
- OAI rate
- Warning Letter rate
- Inspections by Industry, Scope, and Inspection Reason
- And much more
Investigator Profiles are not sold as on-demand documents. If you’d like to see one, fill out the form below to connect with our Sales Team and get it in your inbox as little as 24 hours.
Talk to sales to access our investigator profiles
Loading...
Investigator Profiles Are Just the Tip of the Iceberg
Becoming a Redica Systems customer not only provides you with unlimited access to profiles similar to the one above for every current and former FDA Investigator, but also grants you access to:
Inspection Intelligence
- Pre-Approval Inspection (PAI) trends
- The ability to filter nearly any FDA enforcement action by GxP labels like GMP, GCP, etc.
- Latest 483s: see what the trends are across all FDA Investigators
- CFR Heatmap: Citations aggregated by Subpart by Year
Vendor Intelligence
- Full inspection histories for your vendors down to the specific site level
- All of the functionality mentioned above but isolated to just your vendors
Regulatory Intelligence
- Zero in on the trends for a specific agency/regulator (ex. FDA, MHRA, EMA, Health Canada)
- All the latest "Signals" from agencies around the world, segmented by Country, Type (ex. Guidance, Decree...), Category (ex. Rules/Regulations/Guidance, News, Reports...), Theme (ex. Cell and Gene Therapy, AI...), and more