FDA Investigator Tiffani D Wilson
Tiffani D Wilson has conducted inspections on 25 sites in 10 countries as of 17 Nov 2014. See below for a list of the FDA enforcement documents resulting from those inspections.
Investigator Details
Number of Inspected Sites:
25
Last Inspection Date:
17 Nov 2014
Investigator Role:
FDA Investigator
Redica ID:
Countries of Inspections:
United States of America,
Germany,
Sweden,
Iceland,
United Kingdom of Great Britain and Northern Ireland,
Netherlands,
France,
Denmark,
Mexico,
Switzerland
FDA Investigators that have inspected at least one site in common with Tiffani D Wilson:
Akbar J Zaidi,
Alice S Tsao,
Alicia M Mozzachio,
Amanda E Lewin, PhD,
Amy M Cramer,
Anastasia I Offordile,
Anastasia M Shields,
Andrew J Barrowcliff,
Anita R Michael,
Ankur C Patel,
Anna Lazar,
Annemarie B Randow,
Annemarie Bodnar,
Anthony A Charity,
Anthony N Onianwa,
Arindam Dasgupta, PhD,
Azza Talaat,
Barbara Janine Breithaupt,
Barbara Jwilimczyk Macri,
Barbara M Frazier,
Bijoy Panicker,
Brandy N Lepage,
Brooke K Higgins,
Brooke K Seeman,
Bruce H Mccullough,
Byungja E Marciante,
Christina K Theodorou,
Chryste D Best,
Constance Y Fears,
Craig D Zagata,
Cynthia A Palmer,
Cynthia J Lee, MS,
Cynthia L Rakestraw,
Daniel J Deciero,
Daniel J Grabicki,
Daniel J Mechenbier,
Daniel J Min,
Darla J Christopher,
David A Oluwo,
David M Beltran,
Deborah J Parris,
Denise M Digiulio,
Dennis Cantellops Paite,
Diane T Bargo,
Diane T O'brien,
Donald L Lech,
Douglas A Campbell,
Dr. Barbara D Paul, PhD,
Dr. Carmelo Rosa,
Dr. Mark J Seaton, PhD,
Emest F Bizjak,
Erica L Nicoll,
Farhana Khan,
Frederick F Razzaghi,
Gajendiran Mahadevan, PhD,
Gayle S Lawson,
George Pyramides,
Gerald B Seaborn, Jr,
Gianine E Tompkins,
Gwyn G Dickinson,
Hala L Selby,
Hala Lj Whetstone,
Hasan A Irier, PhD,
Helen B Ricalde,
Heriberto Negron Rivera,
Ivis L Negron,
James A Lane,
James M Mason,
James M Odonnell,
James Norman,
James R Birkenstamm,
James W Plucinski,
Jean Blackston Hill,
Jeffrey A Sommers,
Jennifer L Gustavus,
Jessica S Estriplet,
Joanne Hajoway,
Joanne M Hajoway,
Joel D Hustedt,
Joey V Quitania,
John A Podsadowski,
Jorge L Guadalupe,
Jose Acruz Gonzalez,
Jose M Cayuela,
Jose R Hernandez,
Joseph R Hatcher,
Joshua P Wireman,
Julianne C Mccullough,
Junho Pak,
Karen A Briggs,
Karen S Anthony,
Karen Takahashi,
Kendra L Brooks,
Kenneth M Gordon,
Kenneth Nieves,
Kent C Faul,
Ko U Min,
Kristina J Donohue,
Kristy A Zielny,
Lance Mde Souza, MBA,
LCDR Chad N Thompson,
LCDR Margaret Edi Gennaro,
LCDR Susan E Polifko,
LCDR William H Bender,
Liatte Kreuger, PharmD,
Linda M Hoover,
Lisa L Flores,
Lisa M Bellows,
Lisa M Feola,
Lisa Shin,
Lloyd D Payne,
Lori A Holmquist,
Lori B Wikstrom,
Lori S Lawless,
Lori Wikstrom,
Lourdes Valentin Aponte,
LTJG Bradley E Benasutti,
Lucas B Leake,
Luis A Carrion,
Luis A Dasta,
Lynette P Salisbury,
Marcellinus D Dordunoo,
Marcus F Yambot,
Margaret Faulkner,
Maria A Bienkowski,
Maria A Reed,
Maria A Ruttell,
Marie B Buen Bigornia,
Marisol Faberlle,
Mark E Parmon,
Mary R Kirker,
Matthew M Henciak,
Matthew R Noonan,
Meena Bansal Gupta, PhD,
Melba Trivera Clavell,
Melissa J Garcia,
Merideth K Rose,
Michael L Casner,
Michael R Klapal,
Michele Gottshall,
Michele L Obert,
Michele M Falchek,
Monica E Murie,
Monika Borkowska,
Mra Davism,
Mra Mcculloughj,
Mra V Millarw,
Nicholas A Violand,
Patrick C Klotzbuecher,
Paul L Bellamy,
Prabhu P Raju,
Qin Xu,
Rachel C Harrington,
Rafael Nevarez Nieves,
Raquel Gonzalez Rivera,
Rebecca Rodriguez,
Regina T Brown,
Richard H Penta,
Robert J Martin,
Roy R Rinc,
Ruben C Ayala, PharmD,
Russell J Glapion,
Saied A Asbagh,
Saleem A Akhtar,
Sam Pepe,
Sandra A Hughes,
Shirley J Berryman,
Simone E Pitts,
Sneha S Patel,
Stephen J Koniers,
Suzanne M Healy,
Suzanne M Muchene,
Suzanne N Vallez,
Suzanne Vallez,
Taichun Qin, PhD,
Tamil Arasu, PhD,
Tammy L Chavis,
Tammy M Phillips,
Tara G Bizjak,
Tarun D Mehta,
Theresa Kirkham (NMI),
Thomas E Friel,
Thomas J Arista,
Thomas W Gordon,
Thuy T Nguyen, LCDR,
Timothy T Kapsala,
Tonia F Bernard,
Travis R Hunt,
Truong Xuan Nguyen (Andy),
Ucheabuchi Cchudi Nwankwor,
Uttaniti Limchumroon (Tom),
Vesna V Stanoyevitch,
Vlada Matusovsky,
Walter L Fava,
Wanda Y Fields,
Wanda Y Honeyblue,
Wendy G Tan, PhD,
Xikui Chen (nmi), PhD,
Yong Hu,
Yvesna C Blaise,
Yvette I Henry,
Yvette I Johnson,
Yvins Dezan,
Yvonne C Mcknight
Tiffani D Wilson's Documents
| Publish Date | Document Type | Title |
|---|---|---|
| March, 2007 | EIR | Calumet Karns City Refining LLC - EIR, 2007-03-27 |
| November, 2002 | EIR | Charles River Laboratories, Inc. - EIR, 2002-11-25 |
| November, 2002 | FDA 483 Response | Charles River Laboratories, Inc. - Form 483R, 2002-12-19 |
| November, 2002 | FDA 483 | Charles River Laboratories, Inc. - Form 483, 2002-11-25 |
| December, 2014 | FDA 483 | KVK-TECH, INC - Form 483, 2014-12-11 |
| December, 2011 | FDA 483 | Sigmapharm Laboratories, LLC - Form 483, 2011-12-22 |
| February, 2004 | FDA 483 | Actavis Mid-Atlantic, LLC. - Form 483, 2004-02-23 |
| December, 2010 | FDA 483 | ARx, LLC - Form 483, 2010-12-03 |
| April, 2008 | EIR | AstraZeneca AB - EIR, 2018-05-08 |
| December, 2014 | EIR | KVK-TECH, INC - EIR, 2014-12-11 |
Experience Redica Systems’ NEW investigator profiles and dashboards
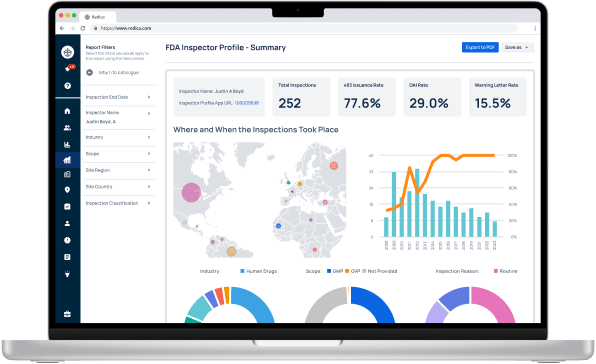
Redica Systems customers get access to over 1,400 FDA Investigator profiles, containing powerful analysis like:
- Total inspections conducted
- 483 rate
- OAI rate
- Warning Letter rate
- Inspections by Industry, Scope, and Inspection Reason
- And much more
Investigator Profiles are not sold as on-demand documents. If you’d like to see one, fill out the form below to connect with our Sales Team and get it in your inbox as little as 24 hours.
Talk to sales to access our investigator profiles
Loading...
Investigator Profiles Are Just the Tip of the Iceberg
Becoming a Redica Systems customer not only provides you with unlimited access to profiles similar to the one above for every current and former FDA Investigator, but also grants you access to:
Inspection Intelligence
- Pre-Approval Inspection (PAI) trends
- The ability to filter nearly any FDA enforcement action by GxP labels like GMP, GCP, etc.
- Latest 483s: see what the trends are across all FDA Investigators
- CFR Heatmap: Citations aggregated by Subpart by Year
Vendor Intelligence
- Full inspection histories for your vendors down to the specific site level
- All of the functionality mentioned above but isolated to just your vendors
Regulatory Intelligence
- Zero in on the trends for a specific agency/regulator (ex. FDA, MHRA, EMA, Health Canada)
- All the latest "Signals" from agencies around the world, segmented by Country, Type (ex. Guidance, Decree...), Category (ex. Rules/Regulations/Guidance, News, Reports...), Theme (ex. Cell and Gene Therapy, AI...), and more